Are you planning to use a UV visible spectrophotometer for your scientific research or experiments? Before diving into the measurements, it is important to have a good understanding of the instrument and its proper usage. A UV visible spectrophotometer is a scientific instrument that measures the absorption and transmission of light in the ultraviolet (UV) and visible (Vis) range of the electromagnetic spectrum. This instrument is widely used in various fields such as chemistry, biochemistry, and materials science to study the properties of substances and molecules. However, incorrect use of the instrument can lead to inaccurate results and unreliable data. In this guide, we will provide a comprehensive overview of the things you should consider before using a UV visible spectrophotometer. By following these guidelines, you can ensure accurate measurements and obtain reliable data for your research.

Principle of a UV Visible Spectrophotometer
The principle of UV visible spectrophotometer is based on the measurement of the absorption or transmission of light by a sample in the ultraviolet (UV) or visible (Vis) range of the electromagnetic spectrum. The spectrophotometer measures the intensity of light that passes through a sample at a specific wavelength, and compares it to the intensity of the incident light.
The ultraviolet-visible absorption spectrum is a molecule or group in a substance that absorbs the energy of the incident ultraviolet-visible light to produce a characteristic banded spectrum.
The absorption spectrum can be used to identify the presence of specific molecules in a sample, determine the purity of a substance, and measure the concentration of a sample.
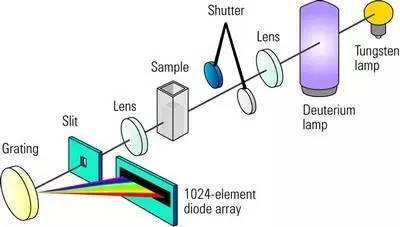
The Beer-Lambert law describes the relationship between the concentration of a sample, the path length of the sample, and its absorbance.
That is, A=εbc, (A is the absorbance, ε is the molar absorptivity coefficient, b is the thickness of the liquid pool, and c is the concentration of the solution).
Then the solution can be quantitatively analyzed. According to this law, the absorbance of a sample is directly proportional to its concentration and the path length of the sample. The relationship between the concentration and the absorbance of a sample can be used to determine the concentration of an unknown sample.
How to Set UV Visible Spectrophotometer?
To set up a UV visible spectrophotometer for measurements, you need to follow several important steps. Here is a general guide for UV visible spectrophotometer settings:
Instrument calibration: Before taking measurements, you should calibrate the instrument to ensure accurate and reliable results. Instrument calibration involves checking the performance of the instrument by measuring a standard reference material. This allows you to detect any instrument drift and correct it before taking measurements.
Adjusting the slit width: The slit width controls the amount of light entering the spectrophotometer. A wider slit allows more light to enter, while a narrower slit provides higher resolution. You should adjust the slit width according to the sample being measured, balancing the need for high resolution with sufficient light intensity.
Choosing the appropriate measurement mode: UV visible spectrophotometers can operate in different measurement modes such as absorbance, transmittance, or reflectance. You should choose the appropriate measurement mode based on the sample and the information you want to obtain.
Choosing the appropriate wavelength: UV visible spectrophotometers allow you to select a specific wavelength of light to pass through the sample. You should choose the appropriate wavelength based on the sample properties and the information you want to obtain. Some instruments have an automatic wavelength selection function, while others require manual selection.
Sample placement: Careful placement of the sample in the spectrophotometer is critical for obtaining accurate measurements. The sample should be placed in a clean and dry cuvette, and the cuvette should be positioned properly in the sample holder to ensure that the light path is unobstructed.
By following these steps, you can ensure that your UV visible spectrophotometer is set up correctly for accurate and reliable measurements. It is important to always refer to the instrument manual for specific instructions on setting up and using your particular instrument.

What Is the Limitation of a UV visible Spectrophotometer?
UV-Visible spectrophotometry is a powerful analytical technique used in various fields, including chemistry, biology, physics, and environmental science. However, it also has some limitations, including:
Limited range: UV-Visible spectrophotometers are useful only for the analysis of compounds that absorb light in the UV-Visible region. If a sample does not absorb light in this region, it cannot be analyzed using this technique.
Interference: Some compounds may interfere with the analysis by absorbing light in the same region as the compound of interest. This interference can lead to inaccurate results.
Dilution: Samples may need to be diluted to obtain accurate readings, which can affect the accuracy of the results.
Solubility: The sample must be soluble in the solvent used for analysis. If the sample is not soluble, it cannot be analyzed using this technique.
Instrument limitations: The quality of the data obtained is directly related to the quality of the instrument. The sensitivity, accuracy, and precision of the instrument are critical factors that can affect the accuracy of the results.
Sample preparation: The preparation of the sample is essential to obtain accurate results. If the sample is not properly prepared, it can lead to inaccurate results.
Overall, UV-Visible spectrophotometry is a valuable analytical technique that has some limitations, but with careful sample preparation and analysis, accurate and precise results can be obtained.