Today, this article will introduce the XRD application in material analysis. You will learn the definition of XRD analysis, 5 directions of XRD application, and 3 XRD application cases.
Introduction of XRD Analysis
XRD (X-ray diffraction) analysis is a structural analysis method that uses X-ray diffraction formed by crystals to analyze the spatial distribution of internal atoms in a substance. Bragg equation:
2dsinθ=nλ, (n=1,2…)
Among them: d is the crystal plane spacing; θ is the angle between the incident X-ray and the corresponding crystal plane; Diffraction occurs only when it is n times larger.
Application of X Ray Diffraction
1. Phase Analysis of XRD Application
Each crystal has its interplanar spacing d, and the atoms are arranged in a certain way, which reflects that the spectral lines of various crystals have their specific positions on the diffraction pattern. Number and Intensity are I. Therefore, it is only necessary to compare the angle and intensity of each spectral line in the diffraction pattern of the unknown with the spectral lines of known samples to achieve the purpose of XRD analysis.
By performing X-ray diffraction on the material and analyzing its diffraction pattern, information such as the composition of the material, and the structure or morphology of the atoms or molecules inside the material can be obtained.

2. XRD Application – Determination of Grain Size
The determination of grain size by X-ray Diffraction is based on the phenomenon that the width of the diffraction line is related to the grain size of the material. For TiO2 nanopowder, its main diffraction peak 2θ is 21.5°. When the copper target is used as the diffraction source, the wavelength is 0.154nm, the 2θ of the diffraction angle is 25.30°, the measured full width at half maximum is 0.375°, and the general Scherrer constant is 0.89. According to the Scherrer formula, the grain size can be calculated.
In addition, according to the grain size, the specific surface area of the nanopowder can also be calculated.
3. Small Angle X-ray Diffraction of XRD Application
In the nano-multilayer film material, two thin film layer materials are repeatedly overlapped to form a modulation interface. When X-rays are incident, the well-periodic modulation interface will be the same as the crystal plane parallel to the film surface. When the Bragg condition is satisfied, coherent diffraction will occur, forming sharp diffraction peaks.
Since the modulation period of the multilayer film is much larger than the maximum interplanar spacing of metals and compounds, only the X-ray Diffraction peaks produced by the modulation interface of the small-period multilayer film can be observed in small-angle diffraction. While the large-period multilayer film modulation, the interface X-ray Diffraction peaks cannot be followed because of the smaller diffraction angle.
Therefore, the small-angle X-ray Diffraction method can be used to determine the amplitude modulation period of a well-prepared small-period nano-multilayer film.
4. XRD Application – Determination of Film Thickness and Interface Structure
With the rapid development of nanomaterials, the study of nanofilms is becoming more and more important. The use of X-ray Diffraction to study the thickness and interface structure of thin films is also an important direction for the development of X-ray Diffraction. Through two-dimensional XRD diffraction, the longitudinal depth analysis results of the phase can also be obtained, and the phase distribution results of the interface can also be obtained.
5. XRD Application – Identification of the State of Matter
Different states of matter have different diffraction effects on X-rays, so X-ray spectra can be used to distinguish crystalline and amorphous states. Generally, the X-ray Diffraction spectrum of amorphous substances is a straight line, and the XRD of diffuse peaks that appear at low 2θ angles is generally composed of liquid solids and gas solids.
Crystalline substances can be divided into microcrystals and crystalline states. Microcrystals have the characteristics of crystals, but due to the small crystal grains, the diffraction peaks will be broadened and dispersed. At the same time, well-crystallized crystalline substances will produce sharp diffraction peaks.
XRD Application Cases in XRD Analysis
- Qualification of a single substance
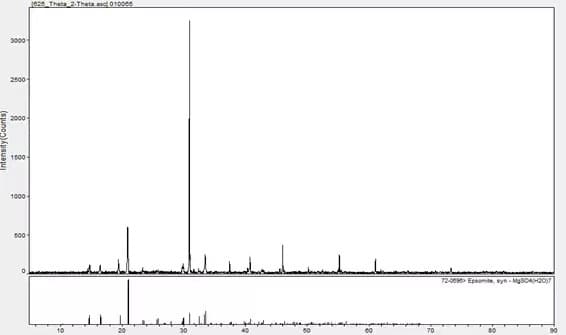
A single substance can be qualified by X-ray Diffraction, and the matching result is magnesium sulfate heptahydrate.
- Qualitative Mixture
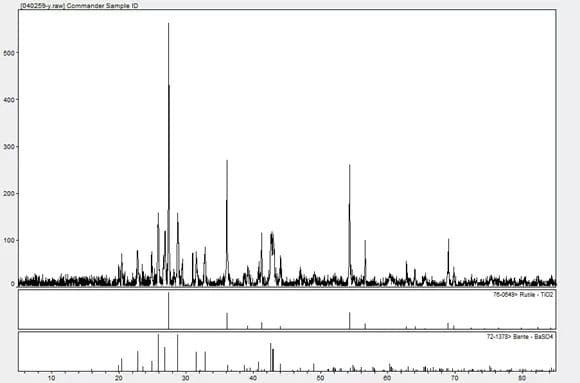
The mixed substance can be qualified by X-ray Diffraction, and the matching results are titanium dioxide and barium sulfate.
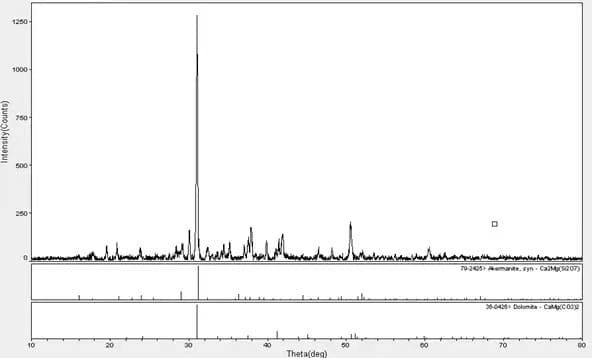
The X-ray Diffraction test of an adhesive’s ash content shows that the adhesive fillers are calcium carbonate, magnesium carbonate, and silicate.
3. Determination method of the glass body content of slag powder
The vitreous content is obtained by measuring the ratio of the area of the vitreous body part in the X-ray diffraction pattern of the granulated blast furnace slag powder to the area on the bottom line.
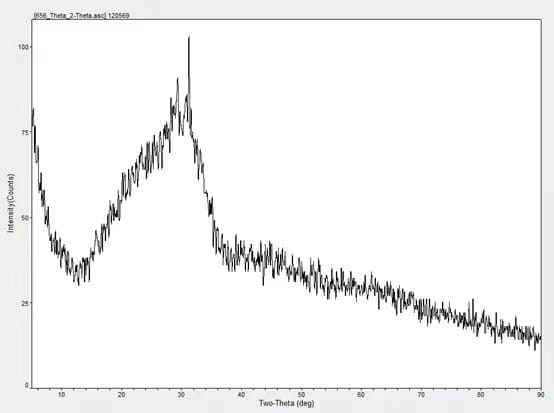
The vitreous body content was measured by XRD, and the content was 97.80%.



