At present, the fluorescence analysis method has developed into an important and effective spectrochemical analysis method, and the fluorescence spectrometer like fluorescence spectrophotometer has also been widely used. Fluorescence is a type of radiative transition, which is the radiation released when a substance is deactivated from an excited state to a low-energy state with the same multiplicity.

Characteristics of fluorescence analysis
You need to know the feature of fluorescence analysis before you learn the principle and application of the fluorescence spectrometer.
1. The main characteristics of fluorescence analysis are high sensitivity and good selectivity, and the sensitivity of fluorescence analysis is 2-3 orders of magnitude higher than that of absorption spectrum measurement. Spectrophotometry is usually on the order of 10-7, while fluorescence has a sensitivity of 10-9.
2. Strong selectivity. Fluorescent substances have two characteristic spectra: excitation spectrum and absorption spectrum. Compared with the single absorption spectrum of spectrophotometry, the fluorescence spectrum can identify substances based on the excitation spectrum and emission spectrum.
3. The amount of information is rich, and various parameters of fluorescent substances can be provided.
4. However, the fluorescence analysis method also has its shortcomings: ① many substances do not fluoresce. ② the relationship between the generation of fluorescence and the structure of the compound is not clear. ③ there are many interference factors, such as photolysis, oxygen quenching, and easy pollution.
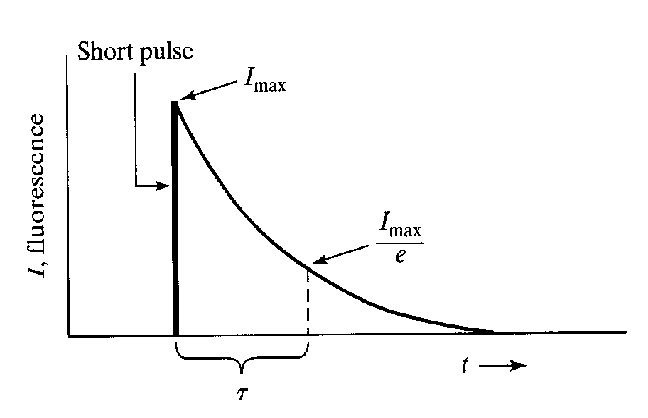
Principle and components of a fluorescence spectrometer
The fluorescence spectrometer records the relationship between the emitted light intensity and the characteristics of the excitation source, sample characteristics, temperature, time, space, energy, and other related characteristics by selecting a suitable detector and working mode when the experimental sample is excited. In this way, the luminescent process can be better studied or utilized.
Fluorescence spectrometer components: light source, excitation wavelength monochromator, sample chamber, emission wavelength monochromator, detector, and control unit.
4 main application areas of a fluorescence spectrometer
1. Application in the field of biology
This field is mainly used for clinical determination of the content of certain components in biological samples, analysis of biotechnology, and immune technology, such as the determination of deoxyribose and deoxyribonucleic acid, DNA, antibodies, antigens, and other aspects of research. In this field, various fluorescent probes are mainly used for analysis and detection, which are mainly divided into biological nano-fluorescent probes and biological non-nano fluorescent probes.
2. Application in the food field
This field is mainly used for the analysis and detection of minerals and metal elements, amino acids, vitamins, fungal pollution, additives, preservatives, harmful substances in food packaging, pesticide residues, etc. in food. Especially the combination with HPLC, TLC, FIA, and other technologies can better achieve the detection effect of various substances in food.
3. Application in drug analysis
In the field of drug analysis, fluorescence analysis can be used to identify active ingredients of drugs, study pharmacokinetics, and analyze clinical pharmacology and pharmacodynamics. At present, it is widely used in the analysis of antibiotic drugs, analgesics, sedatives, hemostatic drugs, etc.
4. Application in environmental analysis
This field mainly uses fluorescence analysis to detect the content of substances in the environment, mainly for water, ore, and soil.
Application of fluorescence spectrometer
The application of fluorescence spectrometers includes the application of metal-organic complex luminescent materials, fluorescent probes, molecular sensors, detection of substances, light-emitting devices, rare earth-doped luminescent materials, etc.
1. Applications of metal-organic complex luminescent materials
Many transition metals can form new energy-level structures (HOMO/LUMO) when they cooperate with ligands. The transition between the ground state and these energy levels can easily lead to a significant increase in quantum yield relative to the ligand. At the same time, it can be achieved by changing the coordination center. The ligand structure modulates its emission band over a wide band.
(1) Intra-Ligand Charge Transfer (ILCT)
(2) Ligand-to-metal charge transfer (LMCT)
(3) Metal-to-ligand charge transfer (MLCT)
(4) Ligand-to-ligand charge transfer (LLC)
(5) Metal-metal to ligand charge transfer (MMLCT), etc.
Metal ions glow
f-f transition: Sm3+, Eu3+, Tb3+, Dy3+, Tm3+, Nd3+, Er3+, Yb3+, etc.
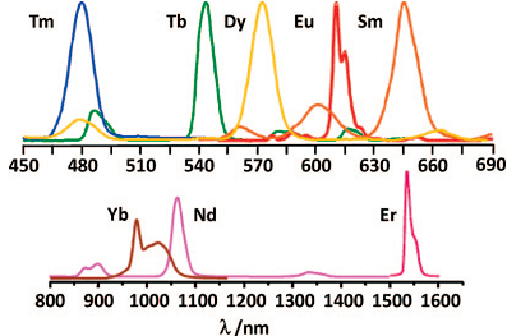
2. Application of rare earth doped luminescent materials
This application includes rare earth-doped laser crystals and rare earth-doped nanomaterials.
(1) Both rare earth ions and semiconductor nanocrystals are good luminescent materials.
(2) The luminescence of rare earth ions can be sensitized by the exciton recombination of semiconductors, which greatly enhances the luminescence efficiency of rare earth ions.
(3) By controlling the size of the synthesized semiconductor nanocrystals to tailor the band gap of the semiconductor to match the excited state energy level of the luminescent center, to achieve efficient energy transfer or luminescence.
(4) Rare earth ions are the best spectroscopic probes sensitive to the structure. Experiments can be designed to detect the room temperature structural phase transition process of some small semiconductor nanocrystals in situ and analyze their internal mechanism.
(5) Rare earth-doped semiconductor nanomaterials have broad application prospects in green lighting sources, nano-optoelectronic devices, flat panel displays, nano-biomarkers, and sensors.
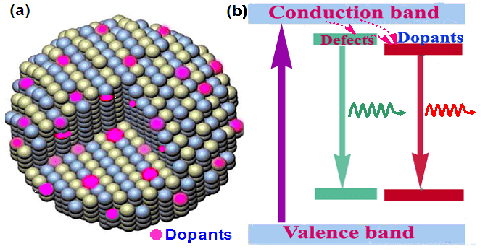
2. Application of Fluorescent Probes
Fluorescent probe: A small molecular substance that changes one or several fluorescent properties by non-covalent interaction with proteins or other macromolecular structures. It can be used to study the properties and behavior of macromolecular substances.
Currently commonly used fluorescent probes include fluorescein probes, inorganic ion fluorescent probes, fluorescent quantum dots, and molecular beacons. In addition to the quantitative analysis of nucleic acid and protein, fluorescent probes are widely used in nucleic acid staining, DNA electrophoresis, nucleic acid molecular hybridization, quantitative PCR technology, and DNA sequencing.
4. Detection of substances
(1) High sensitivity
2 to 4 orders of magnitude higher than UV-Vis spectrophotometry.
Lower detection limit: 0.1~0.1mg/cm-3.
Relative sensitivity: 0.05mol/L quinine bisulfate in sulfuric acid solution.
(2) strong selectivity
It can not only be based on the characteristic emission spectrum but also according on the characteristic absorption spectrum.
(3) Small amount of sample
Disadvantages: the scope of the application is small.