In the work of analyzing test substances, the pretreatment of samples is a very complicated problem. It is one of the most important links in the analysis and detection work, and it is also one of the main ways for us to analyze the source of detection errors. Therefore, this paper summarizes four basic pretreatment methods for samples in detection work by atomic absorption spectrometry. At the same time, we will discuss the basic detection methods of 6 types of samples in routine analysis and detection. These methods are all feasible and can be referenced by AAS (Atomic absorption spectrophotometer) users.

The 4 most important basic methods in AAS general sample pretreatment
1. Wet digestion method
When the sample is about 0.1000 ~ 0.5000g, the commonly used mixed acid, and the ratio when used are:
(1) HNO3:HCLO3=5:1
(2) HNO3: H2SO4=5:1
(3) HNO3:HCl=5:1
(4) Pure HNO3
Note: volatile (acetone, ether, ethanol, etc.) and flammable and explosive substances cannot be present in the sample during digestion.
The wet digestion method is widely used, and everyone is very familiar with it. The specific operation will not be repeated here.
2. Dry ashing method
The sample size is generally 2.000 ~ 5.000g, and the treatment method is not easy to volatilize:
The sample is placed in a porcelain crucible, first add a few drops of water to wet, add a bit of concentrated nitric acid, heat and carbonize in a small fire, and then transfer it to a muffle furnace for ashing at about 550℃ for 2 to 4 hours. After cooling, take it out (colorless or light color), and dissolve it with other acids plus HNO3 (1:1), it varies with different samples, filter, makeup to volume, set 10mL, 25mL, and 50mL for use.
3. High-pressure tank method (polytetrafluoroplastic made of the lidded tank)
When the sample is less than 0.3000g, add 6 mL of mixed acid, add 1mL of HF(H2O2), close the lid of the autoclave, heat at 160°C for 5 hours, cool, filter, and set to volume for later use.
4. Microwave digestion method
Mixed acids commonly used in general work are:
(1) HNO3: HCLO3
(2) HNO3: H2SO4
(3) Pure HNO3
Note: Which acid is used for specific digestion should vary with different samples. Readers are invited to choose as appropriate.
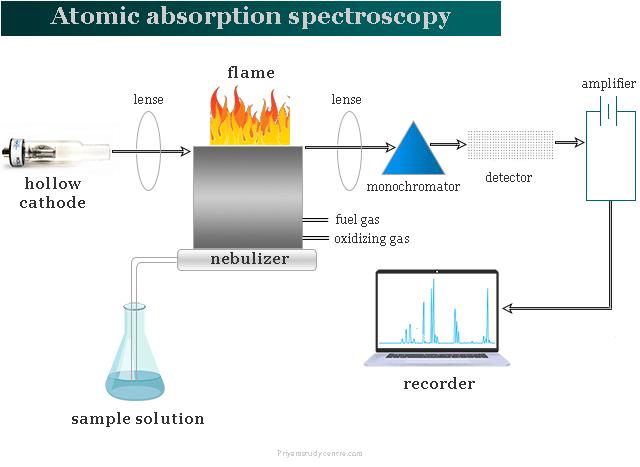
AAS analysis and detection method of conventional samples
1. Test of Pb, Cd, As, Mo, Cr, etc. (graphite furnace AAS method)
Pb: sample 1.0mL and dilute to 10mL with 1% HNO3 to be tested. Linear range 0~20ng/mL, drying temperature 80~100℃, ashing temperature 200℃, atomization temperature 1500℃
Cd: sample 1.0mL and dilute to 10mL with deionized water to be tested. Linear range 0.1~0.4ng/mL, drying temperature 80~100℃, ashing 200℃, atomization 1800℃
As: sample 1.0mL, add 100μL Ni (2mg/mL) and dilute to 10mL with 1% HNO3 to be tested. The linear range is 0~4ng/mL, the drying temperature is 80~100℃, the ashing temperature is 200℃, and the atomization temperature is 2200℃.
Mo: (soil pH greater than 7.5 will affect the absorption of Mo by plants. Do not dilute the sample with HCL, otherwise Mo CL2 will be produced and volatilized):
A sample of 1.0mL was diluted with 1% HNO3 to 10mL to be tested. Using Pd as the modifier, the linear range is 0-20ng/mL, the drying temperature is 80-100°C, the ashing temperature is 1200°C, and the atomization temperature is 2600°C.
Cr: Sampling 1mL, dilute to 100mL with deionized water, and sample for testing as appropriate. Linear range 0~40ng/mL, drying temperature 80~100℃, ashing 1000℃, atomization 2600℃

2. Analysis and testing of Se, K, Na, Ca, Mg, etc. in plant samples
Wash and dry the plant samples, fix them at 150°C for 10 minutes, then dry them at 70°C, and crush them for visual inspection.
(1) Test of Se in forage grass: sample 1 mL plus improver Ni(NO3) 2 100μL (3mg/mL), dilute to 10mL with 1% HNO3 0.1% Triton (1:1), and sample as appropriate test. The linear range is 0~20ng/mL, the drying temperature is 80~100℃, the ashing temperature is 1000℃, and the atomization temperature is 2400℃.
(2) Test of K and Na in plants: Weigh 0.2000g of the sample into a polyethylene bottle, add 10mL of mixed acid, extract in a constant temperature water bath at 90°C for a certain period, then filter to a volume of 50mL, and sample and test as appropriate.
(3) Test of Ca and Mg in plants: The sample is incinerated at 550°C, dissolved with hydrochloric acid (1+1) and the volume is adjusted to 50mL (5% CsCl2 modifier 2 mL) for sampling and testing as appropriate.
3. Test method for Be in mushrooms and tea (transverse heating AAS method can be used)
The sample was washed and wiped dry, and the green was fixed at 105°C, dried at 70°C, crushed after cooling, and passed through 60 mesh for use.
Weigh 1.0000g of sample into a 150mL beaker, add 15mL of HNO3, 1mL of H2SO4, cover with a watch glass, digest at low temperature, remove and cool when white smoke occurs, dilute to 25mL, and sample for testing as appropriate. Test results: The linear range of Be is 0~8ng/mL. Instrument conditions during the test: drying temperature 130℃, ashing temperature 1500℃, atomization temperature 2300℃.
4. Ge test in beverages (transversely heated flat graphite tubes can be used)
Test results: The linear range can reach 0~200ng/mL, Ni(NO3)2 improver 50μg/mL, 1% HNO3 medium.
Instrument conditions: drying temperature 130°C, ashing temperature 800°C, atomization temperature 2000°C.
5. Ca detection in steel slag (generally by flame AAS method)
The sample is over 200 mesh, weigh 0.0500g into a 50mL PTFE crucible, first wet it with a small amount of water, use water: nitric acid: hydrofluoric acid = 5:8:6, heat it to complete dissolution, add 2mL of sulfuric acid, continue to heat until white smoke emerges, and make up to 50mL with water for use. Because steel slag generally contains niobium, it interferes with the analysis of calcium. Add improver Triton-100 (20%), Vc (0.1 M/L) 2% HNO3, and the effect is very good.
6. Determination method of some metal elements in blood samples
Human blood contains various trace substances. For example inorganic salts, various metabolites, O2, hormones, enzymes, antibodies, trace elements, etc. These trace substances can generally be detected by various methods. In particular, heavy metal elements that are beneficial, toxic, and harmful to the human body can be detected by AAS. For specific digestion methods, see “2, 3), (3)”. E.g:
(1) Determination of Cu in serum (flame AAS method): take 0.8mL of serum, dilute it with 1% HNO3 to 10mL, and then sample and test.
(2) Determination of As in serum (graphite furnace AAS method): Dilute with 1% HNO3 and test with Ni(NO3)2 as matrix modifier.
(3) Determination of Cd in serum (graphite furnace AAS method): take 2mL of serum into a 50mL beaker, slowly add 1mL of HNO3, leave it for 10 minutes, add 0.5mL of H2O2, and heat it on an electric hot plate for 20 minutes, then add 1mL of HNO3. Add 0.5mL of H2O2 until light yellow, add 4mL of deionized water to dissolve, and sample for testing as appropriate.
(4) Determination of Fe and Cu in albumin (flame AAS method): Take 1mL of sample, add 7mL of mixed acid to a 50mL beaker, put the lid on it, and heat it on an electric hot plate (150°C) until white smoke is emitted. Then set the volume to 25mL, and take samples for testing as appropriate.
(5) Determination of germanium in whole blood: (graphite furnace AAS method): take 1.0mL of venous blood, dilute 4 times with 0.2% Triton-100, and inject 20μL. To improve the sensitivity, a mixture of strontium (30μg) + ammonium nitrate (10μg) was used as a matrix modifier. The ashing temperature was 1000°C and the atomization temperature was 2500°C during the test.

