Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) is a widely used analytical technique for detecting and quantifying trace elements across diverse sample types. The accuracy of ICP-OES results is heavily reliant on effective calibration methods. This article delves into the essential calibration techniques for achieving high precision and accuracy in ICP-OES analysis.

Why Calibration Matters in ICP-OES
Calibration in ICP-OES links the instrument’s measured signal intensity to the actual concentration of elements in a sample. Proper calibration:
- Ensures accurate and reliable measurements.
- Accounts for signal variations due to instrumental or matrix-related factors.
- Meets regulatory and quality assurance requirements.

Primary Types of Calibration Methods in ICP-OES
Different calibration methods address varying sample complexities, matrix interferences, and instrument conditions.
1. External Standard Calibration
External standard calibration is the most common method used in ICP-OES. It involves creating a calibration curve by measuring the signals of a series of standard solutions with known analyte concentrations.
Key Features:
- A series of standards with increasing concentrations is prepared.
- The instrument response (intensity) is plotted against analyte concentration.
- Unknown sample concentrations are determined based on the calibration curve.
Advantages:
- Straightforward and easy to implement.
- Ideal for simple matrices with minimal interferences.
Limitations:
- Prone to errors from matrix effects if the sample matrix differs significantly from the standards.
- Requires precise preparation of calibration solutions.
2. Internal Standard Calibration
This method uses an internal standard—a known quantity of an element not present in the sample or standards. It compensates for signal fluctuations caused by variations in sample introduction, plasma stability, or matrix effects.
Key Features:
- The internal standard is added to all calibration standards and unknown samples.
- Analyte signals are normalized against the internal standard signal.
Advantages:
- Compensates for instrument drift and signal variability.
- Reduces the impact of matrix interferences.
Limitations:
- Selecting an appropriate internal standard is critical; it should have similar physical and chemical properties to the analytes.
- Requires consistent addition of the internal standard.
3. Standard Additions Method
This method is particularly useful for samples with complex matrices. It involves spiking the sample with known concentrations of the analyte and measuring the response.
Key Features:
- The original sample is analyzed without spiking to establish the baseline signal.
- Increasing concentrations of the analyte are added (spiked) to the sample.
- A calibration curve is generated, and the original analyte concentration is determined by extrapolating to zero signal.
Advantages:
- Eliminates errors caused by matrix effects.
- Highly accurate for samples with unknown or variable matrices.
Limitations:
- Time-consuming due to the need for multiple spiked solutions.
- Requires precise spiking and reproducible measurements.
4. Multi-Point Calibration
Multi-point calibration involves using several standard solutions to generate a calibration curve that spans the range of expected analyte concentrations.
Key Features:
Typically requires at least 3–5 standards covering low to high concentrations.
The instrument response is fitted to a linear or non-linear curve, depending on the signal behavior.
Advantages:
- Ensures high accuracy across a wide concentration range.
- Compensates for potential non-linearities in the instrument response.
Limitations:
- Requires more time and effort to prepare multiple standards.
- Not suitable for applications requiring rapid analysis.
5. Single-Point Calibration
Single-point calibration uses only one standard solution with a known concentration to establish a relationship between signal intensity and analyte concentration.
Key Features:
- The intensity of the single standard is compared directly to the sample.
- Assumes a linear relationship between concentration and intensity.
Advantages:
- Quick and simple, suitable for high-throughput analysis.
- Useful for situations where concentration ranges are narrow.
Limitations:
- Less accurate than multi-point calibration.
- Assumes perfect linearity, which may not always be the case.
6. Matrix-Matched Calibration
This approach involves matching the matrix composition of calibration standards to that of the samples. It helps reduce matrix-induced interferences.
Key Features:
- Standards are prepared with similar matrix components (e.g., acids, salts, or organic solvents) as the samples.
- Matrix-matched blanks are also used to correct baseline signals.
Advantages:
- Reduces the impact of matrix effects on analyte signals.
- Provides accurate results for complex sample matrices.
Limitations:
- Preparing matrix-matched standards can be challenging.
- Time-consuming and may require additional reagents or preparation steps.
7. Calibration with Certified Reference Materials (CRMs)
CRMs are highly accurate, traceable materials with known concentrations of analytes. They are used for direct calibration or to validate other calibration methods.
Key Features:
- CRMs are analyzed to ensure the accuracy of calibration curves.
- They serve as quality control checks during the analysis.
Advantages:
- Provides traceable and reliable results.
- Ideal for regulatory compliance and inter-laboratory comparisons.
Limitations:
- CRMs can be expensive and may not be available for all matrices.
- Requires careful handling to avoid contamination.

Common Challenges and Solutions in Calibration of ICP-OES Analysis
1. Matrix Effects
Matrix effects occur when other substances in the sample influence the emission signal of the target analyte. These effects can lead to signal suppression or enhancement, resulting in inaccurate measurements.
Solutions:
- Matrix Matching: Prepare calibration standards with a matrix composition similar to the sample (e.g., acids, salts, or organic solvents).
- Standard Additions Method: Use the standard additions technique to eliminate matrix interferences by spiking the sample with known concentrations of the analyte.
- Internal Standards: Add an internal standard to normalize the signal and compensate for matrix-induced variability.
2. Instrumental Drift
Instrumental drift, caused by fluctuations in temperature, plasma stability, or detector sensitivity, can reduce the accuracy of calibration over time.
Solutions:
- Frequent Recalibration: Perform recalibration at regular intervals to ensure instrument consistency.
- Internal Standards: Use internal standards to track and compensate for signal drift.
- Automated Drift Correction: Many modern ICP-OES instruments have automated systems to monitor and adjust for drift.
3. Contamination of Standards or Samples
Contamination during sample preparation for ICP-OES or from the laboratory environment can lead to false readings and distorted calibration curves.
Solutions:
- Use High-Purity Reagents: Prepare standards using high-purity reagents and deionized water.
- Clean Equipment: Regularly clean sample introduction systems (e.g., nebulizers, spray chambers, and torches) to prevent contamination.
- Monitor Blanks: Regularly analyze blank samples to identify potential sources of contamination.
4. Non-Linear Calibration Curves
Non-linear relationships between analyte concentration and emission intensity can complicate the generation of accurate calibration curves, especially at high concentrations.
Solutions:
- Dilution of Standards: Dilute high-concentration standards to maintain a linear response.
- Use Multi-Point Calibration: Generate a calibration curve using multiple standard solutions across the expected concentration range.
- Non-Linear Fitting: Use advanced curve-fitting techniques if the response is inherently non-linear.
5. Signal Overlap and Spectral Interferences
Spectral interferences arise when emission lines from different elements overlap, making it difficult to resolve the target analyte’s signal.
Solutions:
- Select Alternate Wavelengths: Choose less crowded wavelengths for the target analyte.
- Use Advanced Detectors: High-resolution detectors can distinguish closely spaced spectral lines.
- Mathematical Correction: Apply software-based interference correction algorithms available in modern ICP-OES instruments.
6. Inconsistent Sample Introduction
Variability in the sample introduction system (e.g., inconsistent nebulization or clogging) can lead to erratic signals and poor calibration.
Solutions:
- Routine Maintenance: Regularly clean and inspect nebulizers, spray chambers, and torches for clogging or wear.
- Use an Internal Standard: Compensate for fluctuations in sample introduction by normalizing signals to an internal standard.
- Optimize Operating Conditions: Adjust nebulizer gas flow and sample uptake rates for stable introduction.
7. Stability of Calibration Standards
Calibration standards may degrade over time, leading to inaccurate calibration curves.
Solutions:
- Prepare Fresh Standards: Regularly prepare new standards to ensure their stability and accuracy.
- Store Standards Properly: Keep standards in tightly sealed, contamination-free containers, and store them under appropriate conditions.
- Use Certified Reference Materials (CRMs): Validate calibration accuracy using CRMs with known analyte concentrations.
8. Plasma Instability
Instability in the plasma due to changes in gas flow, power supply, or sample matrix can cause fluctuations in emission intensity.
Solutions:
- Optimize Plasma Parameters: Adjust RF power, gas flow rates, and viewing height to stabilize the plasma.
- Check Torch Alignment: Ensure proper alignment of the torch to maintain consistent plasma conditions.
- Use Robust Plasma Conditions: Select operating conditions that are less sensitive to matrix variations.
Emerging Trends in Calibration for ICP-OES
1. Automation of Calibration Processes
Automated sample preparation and calibration workflows are becoming common, reducing human error and increasing reproducibility.
Key Features:
- Automated dilutors to prepare multi-point calibration curves.
- Robotic systems for consistent sample handling and introduction.
- Integrated monitoring of instrument drift with auto-recalibration triggers.
Impact:
Enhanced precision, time efficiency, and reduced labor-intensive steps.
2. Digital Twin Models for Calibration
Digital twin technology is being used to simulate instrument behavior and optimize calibration strategies without extensive lab trials.
Key Features:
- Predictive calibration based on historical and simulated data.
- Improved detection of non-linear responses through advanced modeling.
- Real-time adjustments in calibration settings based on operational feedback.
Impact:
Higher calibration accuracy with reduced costs in standard consumption.
3. AI-Driven Calibration Optimization
Artificial intelligence (AI) and machine learning algorithms are optimizing calibration curve creation and interference correction.
Key Features:
- AI analyzes complex matrices for efficient calibration standard selection.
- Algorithms correct matrix effects and predict spectral interferences.
- Dynamic recalibration recommendations based on real-time performance data.
Impact:
Improved reliability, especially in complex and high-throughput applications.
4. Use of Nano-Certified Standards
Development of nano-certified reference materials to achieve ultra-trace-level accuracy.
Key Features:
- Better homogeneity and stability at low concentrations.
- Reduced contamination risks during standard preparation.
- Increased compatibility with advanced ICP-OES detectors.
Impact:
Enhanced sensitivity for applications like environmental monitoring and semiconductor analysis.
5. Advanced Software Integration
Advanced ICP-OES spectrometers are integrating advanced software for calibration management.
Key Features:
- Real-time calibration verification and drift monitoring.
- Seamless data transfer to Laboratory Information Management Systems (LIMS).
- Built-in error diagnostics to guide users during calibration.
Impact:
Reduced downtime and consistent data quality across different operators.
6. Matrix-Matched Custom Calibration Kits
Suppliers now offer pre-prepared, matrix-matched calibration kits tailored to specific industries.
Key Features:
- Standards designed for industry-specific matrices (e.g., seawater, petrochemicals).
- Reduction in preparation time and errors.
- Compatibility with automated systems for seamless workflows.
Impact:
Improved consistency across laboratories and applications.
7. Non-Linear Calibration Algorithms
Adoption of non-linear calibration algorithms to address signal saturation and complex emission profiles.
Key Features:
- Curve fitting techniques for non-linear response regions.
- Sophisticated statistical models to enhance curve accuracy.
- Adaptive calibration adjustments based on real-time data.
Impact:
Greater accuracy for high-concentration samples and multi-element analysis.
8. Green Chemistry in Calibration Standards
Focus on sustainable practices in the preparation of calibration standards.
Key Features:
- Use of environmentally friendly solvents and reagents.
- Reduced hazardous waste generation during standard preparation.
- Development of biodegradable and recyclable containers for standard solutions.
Impact:
Lower environmental footprint while maintaining high-quality calibration.
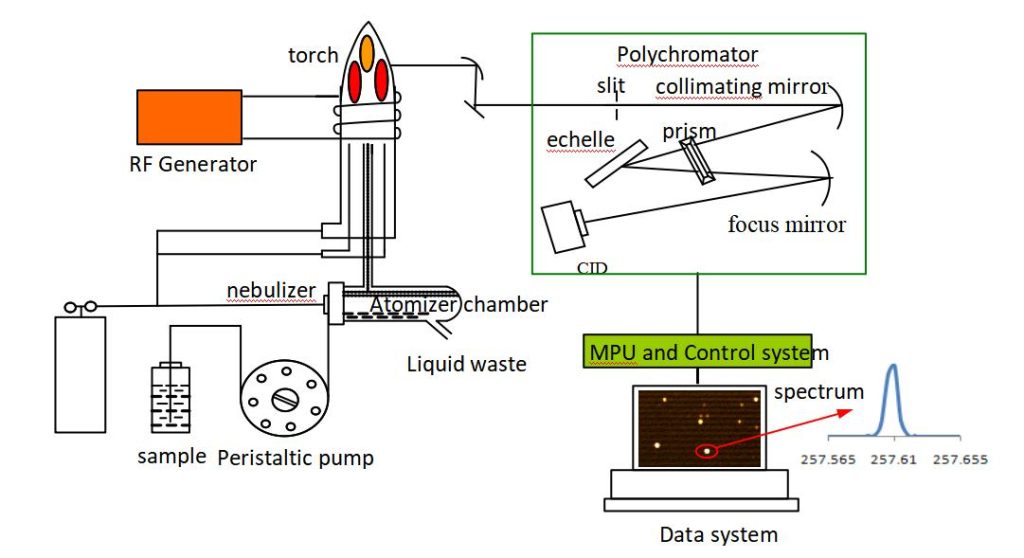
Steps for Effective Calibration in ICP-OES
| Step | Description | Key Tips |
| 1. Define Calibration Goals | Determine the required accuracy, precision, and detection limits for your analysis. | Clearly understand the sample matrix and the elements to be analyzed. |
| 2. Select Calibration Standards | Choose standards with appropriate concentrations and high purity for the target analytes. | Use certified reference materials (CRMs) for reliability. |
| 3. Prepare Calibration Standards | Dilute and prepare standards in a matrix similar to the sample to minimize interferences. | Use deionized water and clean glassware to avoid contamination. |
| 4. Optimize Instrument Settings | Adjust parameters like RF power, nebulizer flow, and viewing height to ensure stable performance. | Perform a warm-up routine for the instrument before calibration. |
| 5. Perform Blank Measurement | Measure the blank to account for baseline noise and contamination. | Ensure blanks are free of analyte contamination. |
| 6. Run Calibration Standards | Measure standards at multiple concentrations to generate a calibration curve. | Use at least 3-5 points, including a zero-concentration point. |
| 7. Verify Calibration Curve | Assess the linearity and correlation coefficient (R² ≥ 0.999 is ideal). | Re-prepare standards if non-linearity is observed. |
| 8. Use Quality Control Samples | Analyze independent QC samples to validate calibration accuracy. | Incorporate matrix-matched QC samples to check for interferences. |
| 9. Monitor Drift and Stability | Check calibration at intervals during analysis to ensure consistent performance. | Recalibrate if significant drift or deviation is detected. |
| 10. Document Calibration Details | Record all calibration parameters, results, and deviations for quality assurance. | Maintain calibration logs for traceability and audits. |

Summary
Employing effective calibration techniques for ICP-OES, such as external standards, internal standards, standard additions, multi-point calibration, etc, alongside regular verification and recalibration, ensures precise analytical results. With advancements in automation and software, the future of ICP-OES calibration is set to become even more efficient and error-free.








2.jpg)