Spectrophotometers and fluorometers are two essential analytical instruments used widely in research, diagnostics, and quality control. While both devices analyze light interactions with samples, they do so using distinct principles and are applied in different contexts. Understanding their differences helps laboratories choose the right tool for specific tasks, ensuring accuracy, efficiency, and cost-effectiveness.
This article explores the key differences between spectrophotometers and fluorometers, focusing on measurement principles, detection ranges, sensitivity, sample requirements, and cost considerations. Hope they will help you understand when to use each instrument in your laboratory.

5 Differences Between Spectrophotometers and Fluorometers
1. Measurement Principle
Spectrophotometer:
A spectrophotometer measures the absorption of light by a sample at specific wavelengths. The instrument directs light through a sample, and a detector measures how much light is absorbed. The absorbance is then used to quantify the concentration of analytes in the sample.
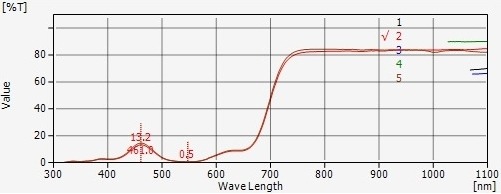
Fluorometer:
A fluorometer, on the other hand, detects the fluorescence emitted by a sample. It shines a light source on the sample to excite specific molecules, which then emit light at a different wavelength. The instrument detects the intensity of the emitted fluorescence, which is proportional to the concentration of the fluorescent compound in the sample.
While a spectrophotometer measures absorbed light, a fluorometer detects emitted fluorescence, making them suitable for different types of analyses.
2. Detection Range and Dynamic Range
Spectrophotometer:
Spectrophotometers generally offer a broad detection range, from UV through visible to near-infrared light. They can measure a wide variety of analytes in liquid and solid samples and handle high-concentration samples well. The dynamic range is typically wide, allowing for accurate measurements across various concentration levels.
Fluorometer:
Fluorometers are optimized for detecting very low concentrations of fluorescent compounds. However, their dynamic range can be narrower than that of spectrophotometers, particularly because of quenching effects at higher concentrations. Fluorometers are ideal for samples where the target analyte is present in low concentrations and emits light.
Spectrophotometers have a broader detection and dynamic range, while fluorometers excel in detecting low-concentration, fluorescent analytes.

3. Sensitivity
Spectrophotometer:
Spectrophotometers offer moderate sensitivity. The sensitivity depends on the optical components and the wavelength used but generally works well for mid-range concentrations of analytes. Spectrophotometers struggle to detect very low analyte concentrations compared to fluorometers.
Fluorometer:
Fluorometers are highly sensitive, often capable of detecting picomolar to nanomolar concentrations of analytes. This sensitivity makes them ideal for detecting trace amounts of fluorescent compounds, where high precision is needed.
Fluorometers are significantly more sensitive than spectrophotometers, making them better suited for low-concentration applications.
4. Sample Type and Preparation
Spectrophotometer:
Spectrophotometers can accommodate a wide variety of sample types, including liquids, solids, and gases. Sample preparation can vary depending on the type of assay being performed but is generally straightforward. Some methods require cuvettes or microplates, and no special treatment is needed if the sample is not highly fluorescent.
Fluorometer:
Fluorometers often require more specialized sample preparation because the analyte must be fluorescent or tagged with a fluorescent molecule. Additionally, fluorescence can be affected by environmental factors like pH, temperature, and solvent, which must be carefully controlled to obtain accurate results.
Spectrophotometers are more versatile in terms of sample type and preparation, while fluorometers require more careful sample handling, especially in ensuring fluorescence.

5. Cost and Complexity
Spectrophotometer:
Spectrophotometers tend to be less expensive than fluorometers, with simpler setups and fewer specialized components. They require less frequent calibration and are easier to operate, making them accessible to a wide range of users.
Fluorometer:
Fluorometers are generally more complex and expensive due to the high sensitivity and specialized optical components required for fluorescence detection. They often require more advanced training for proper use and may involve more maintenance and calibration compared to spectrophotometers.
Spectrophotometers are more affordable and simpler to use, while fluorometers, though more costly and complex, offer advanced capabilities for specific applications.

Spectrophotometer vs Fluorometer: When to Use Each?
The choice between a spectrophotometer and a fluorometer depends on the specific requirements of your application. Here are some guidelines:
| Instrument | When to Use |
| Spectrophotometer | – When measuring absorbance of light |
| – For concentration measurements, purity tests, and kinetics studies | |
| – When handling various sample types (liquids, solids, gases) | |
| – In general labs where moderate to high analyte concentrations are common | |
| – When cost and ease of use are important factors | |
| Fluorometer | – When detecting low concentrations of fluorescent compounds |
| – For fluorescence-based assays in biochemistry, molecular biology, and environmental analysis | |
| – When high sensitivity is crucial, especially for trace analyte detection | |
| – When working with fluorescently labeled samples (e.g., DNA, proteins, toxins) | |
| – In specialized labs where precision and sensitivity are top priorities |
In conclusion, spectrophotometers and fluorometers are both valuable tools for analytical chemistry. Spectrophotometers are versatile and cost-effective for a wide range of absorbance measurements, while fluorometers provide unmatched sensitivity for detecting low concentrations of fluorescent compounds. By understanding their key differences and applications, you can select the most appropriate instrument for your laboratory needs.