Spectroscopy, the study of the interaction between matter and electromagnetic radiation, is a fundamental technique in analytical chemistry. It enables scientists to identify and quantify elements within a sample, making it invaluable across various industries, from environmental monitoring to pharmaceutical research. Among the numerous techniques available, ICP-AES (Inductively Coupled Plasma Atomic Emission Spectroscopy), ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy), ICP-MS (Inductively Coupled Plasma Mass Spectrometry), and AAS (Atomic Absorption Spectroscopy) are the most widely used.
Each method has unique strengths and applications, making the choice of the right spectrometer vital for achieving accurate and efficient results. This article provides an in-depth comparison of these techniques and guides you on when to choose each based on your analytical needs.

Key Comparison Factors
Among ICP-AES, ICP-OES, ICP-MS, and AAS, there are significant differences in the principles, applications, detection limits, etc.. Here are their main differences:
Detection Limits and Sensitivity
| Feature | ICP – AES | ICP – OES | ICP – MS | AAS |
| Principle | Based on atomic emission spectroscopy, atoms are excited by high – temperature plasma to produce characteristic spectral lines | The same as ICP – AES, the name is updated to reflect technological development | Based on mass spectrometry analysis, samples are ionized by plasma and then detected by mass spectrometry | Based on atomic absorption spectroscopy, analysis is carried out through the absorption of light at specific wavelengths of elements |
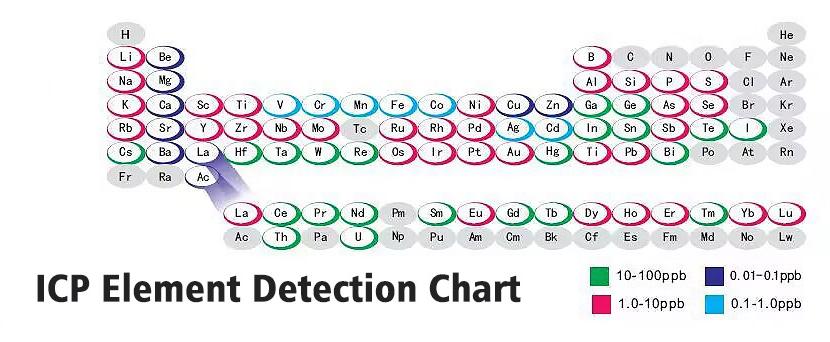
| Detection Limit | For most elements, it is 1 – 10 ppb, and for some elements, it can reach sub – ppb level | Similar to ICP – AES, for most elements, it is 1 – 10 ppb, and for some elements, it can reach sub – ppb level | For most elements, it is at ppt level, and the detection limit is lower than that of ICP – AES | The detection limit is relatively high, usually at sub – ppb to ppm level |
| Analysis Range | It can analyze more than 70 elements, including metals and some non – metals | The same as ICP – AES, it can analyze more than 70 elements, including metals and some non – metals | It can analyze more than 70 elements, including metals and some non – metals, and can also perform isotope analysis | Mainly analyzes metal elements, and some instruments can analyze a few non – metal elements |
| Linear Range | Wide, suitable for the analysis of major and trace elements | Wide, suitable for the analysis of major and trace elements | Wide, suitable for the analysis of major and trace elements, especially good at trace analysis | Relatively narrow, more suitable for the analysis of major elements |
| Interference | Spectral interference is more serious, especially for samples with complex matrices | Spectral interference is more serious, especially for samples with complex matrices | There is less mass spectrometry interference, but there are isobaric interference, etc. | There is relatively less spectral interference, mainly matrix interference |
Cost and Applications
| Feature | ICP – AES | ICP – OES | ICP – MS | AAS |
| Application Fields | Widely used in environmental, food, pharmaceutical, geological and other fields | Widely used in environmental, food, pharmaceutical, geological and other fields | Widely used in environmental, food, pharmaceutical, geological and other fields, especially has advantages in ultra – trace analysis and isotope analysis | Widely used in environmental, food, pharmaceutical, geological and other fields, but not as good as ICP – MS in ultra – trace analysis |
| Equipment Cost | High | High | High, usually higher than ICP – AES and ICP – OES | Low, usually lower than ICP – related equipment |
| Operating Cost | High, with large argon consumption | High, with large argon consumption | High, with large argon consumption and requires high – purity gas | Low, with less gas consumption |

Sample State and Analysis Methods
| Feature | ICP – AES | ICP – OES | ICP – MS | AAS |
| Sample State | Can be powder, block, or solution samples | Can be powder, block, or solution samples | Mainly solution samples, high – salt samples need to be diluted | Mainly solution samples, and some instruments can analyze solid samples |
| Multi – element Analysis | Can perform multi – element analysis simultaneously | Can perform multi – element analysis simultaneously | Can perform multi – element analysis simultaneously and can also analyze isotopes | Usually can only analyze one element at a time, and some instruments can achieve multi – element analysis |
| Qualitative Analysis | Qualitative analysis is carried out by the position (wavelength) of characteristic spectral lines | Qualitative analysis is carried out by the position (wavelength) of characteristic spectral lines | Qualitative analysis is carried out by mass spectrometry, and isotopes can be identified | Qualitative analysis is carried out by the absorption of light at specific wavelengths of elements |
| Quantitative Analysis | Quantitative analysis is carried out by the intensity of characteristic spectral lines, and the standard curve method is generally adopted | Quantitative analysis is carried out by the intensity of characteristic spectral lines, and the standard curve method is generally adopted | Quantitative analysis is carried out by ion counting, and the standard curve method or internal standard method is generally adopted | Quantitative analysis is carried out by the intensity of light absorption, and the standard curve method is generally adopted |

As can be seen from the table above, ICP-MS has obvious advantages in detection limit and isotope analysis, and is suitable for ultra-trace analysis; ICP-AES and ICP-OES perform well in multi-element analysis and constant and trace element analysis, but the equipment and operating costs are relatively high; AAS has advantages in element-specific analysis and lower equipment operating costs, but its detection limit is relatively high and its multi-element analysis capability is limited. In practical applications, it is necessary to select the appropriate analysis technology based on factors such as analysis requirements, sample properties, and budget.
When to Choose ICP-AES, ICP-OES, ICP-MS, or AAS?
When choosing these types of analytical equipment, the following aspects need to be considered:
Analysis Purpose:
If multi – element simultaneous analysis, especially for major and trace elements, is required, ICP – AES and ICP – OES are good choices.
If ultra – trace element analysis or isotope analysis is needed, ICP – MS is more appropriate.
If only one or a few elements need to be analyzed, especially when cost – sensitive, AAS may be a more economical choice.

Sample Properties:
For liquid samples, especially aqueous solutions, ICP – AES, ICP – OES and ICP – MS are all applicable.
If the sample contains high – salt content or a high – concentration matrix, the high – matrix – tolerance ability of ICP – MS may be more important.
For solid samples, AAS may be more suitable, especially for single – element analysis.
Detection Limit Requirements:
If extremely low detection limits are required, such as in environmental monitoring or drug analysis, ICP – MS usually has the lowest detection limit and can meet the analysis requirements at the ppt level.
If the detection limit requirement is at the ppb level, ICP – AES and ICP – OES can also meet the analysis requirements for most elements.
Analysis Speed and Throughput:
If a large number of samples need to be analyzed quickly, the analysis speed of ICP – AES and ICP – OES is relatively fast, and multi – element analysis can be completed in a short time.
Although the analysis speed of ICP – MS is also fast, more pre – treatment steps may be required when dealing with complex – matrix samples, thus affecting the analysis throughput.
Cost Factors:
The equipment and operating costs of ICP – MS are usually high, especially when using high – purity gases and complex sample pre – treatment.
The equipment costs of ICP – AES and ICP – OES are also high, but the operating costs are relatively lower.
The equipment and operating costs of AAS are usually lower than those of ICP – related equipment, especially for single – element analysis.

Application Areas:
In environmental monitoring, food analysis, geological exploration, metallurgy and other fields, ICP – AES, ICP – OES and ICP – MS are all widely used.
In drug analysis, clinical diagnosis, semiconductor material analysis and other fields, the high – sensitivity and isotope – analysis ability of ICP – MS may be more important.
In some cost – sensitive industries, such as small laboratories or educational institutions, AAS may be a more suitable choice.
All in all, when choosing analytical equipment, multiple aspects such as analysis purpose, sample properties, detection limit requirements, analysis speed, cost factors and application areas need to be comprehensively considered. Here is a simple summary:
| Analytical Equipment | Applicable Situations |
| ICP – AES | Multi – element simultaneous analysis, major and trace element analysis, liquid samples, detection limit at ppb level, medium cost |
| ICP – OES | Multi – element simultaneous analysis, major and trace element analysis, liquid samples, detection limit at ppb level, medium cost |
| ICP – MS | Ultra – trace element analysis, isotope analysis, liquid samples, detection limit at ppt level, high cost |
| AAS | Single – element or a few – element analysis, liquid and solid samples, detection limit at sub – ppb to ppm level, low cost |
Selecting the right spectrometer is crucial for achieving accurate, reliable, and efficient elemental analysis. ICP-AES and ICP-OES offer excellent multi-element capabilities, while ICP-MS excels in trace-level precision. AAS remains a cost-effective option for single-element applications. If you need any help of choose these spectrometers, please feel free to contact our engineer. With the right choice, you’ll optimize both analytical performance and operational efficiency.




2.jpg)