The world around us, from the minerals in the earth’s crust to the intricate molecules of pharmaceuticals, is built upon the precise arrangement of atoms in crystalline structures. Understanding these structures is vital for developing new materials, improving existing technologies, and advancing our knowledge of the natural world. X-ray diffraction (XRD) stands as a cornerstone technique in this endeavor, offering a non-destructive method to peer into the atomic lattice and reveal the hidden architecture of crystals. Here we delves into the principles and applications of XRD, illustrating its power in unlocking the secrets of crystal structures.

The Fundamentals of X-ray Diffraction
What are X-rays?
X-rays are a form of electromagnetic radiation with wavelengths in the range of approximately 0.01 to 10 nanometers. This wavelength range is comparable to the interatomic distances in crystals, making X-rays ideal for probing their structure. Discovered by Wilhelm Röntgen in 1895, X-rays have since become indispensable in medical imaging, material science, and crystallography.
When X-rays interact with matter, they can be absorbed, transmitted, or scattered. In XRD, we exploit the scattering phenomenon. X-rays are produced by accelerating electrons onto a metal target, typically copper or molybdenum, generating characteristic X-ray wavelengths. For example, copper K-alpha radiation, a commonly used X-ray source, has a wavelength of approximately 0.154 nanometers. The penetration power of X-rays allows them to interact with the atoms within a crystal, providing a detailed map of their arrangement. This interaction is the foundation of X-ray diffraction for crystal structure determination.
Crystals and their Structure
Crystals are solids characterized by a highly ordered, repeating arrangement of atoms, ions, or molecules. This ordered arrangement forms a crystal lattice, a three-dimensional network of points that represent the positions of these building blocks. The smallest repeating unit of this lattice is called the unit cell, which defines the crystal’s symmetry and dimensions.
There are various crystal systems exist, including cubic, tetragonal, orthorhombic, monoclinic, triclinic, hexagonal, and rhombohedral, each with distinct unit cell parameters and symmetry elements. For example, sodium chloride (NaCl) forms a cubic crystal lattice, where sodium and chloride ions are arranged in an alternating pattern. The unit cell dimensions and the arrangement of atoms within it define the crystal’s macroscopic properties. By understanding the crystal structure, we gain insight into the material’s physical and chemical behavior.
Bragg’s Law
Bragg’s Law, formulated by Sir William Henry Bragg and his son Sir William Lawrence Bragg in 1913, is the foundational principle behind XRD. It states:
nλ=2d sinθ
where:
- n is an integer (the order of diffraction)
- λ is the wavelength of the X-rays
- d is the spacing between the atomic planes in the crystal
- θ is the angle of incidence of the X-rays
This law explains how specific angles of incidence result in strong diffraction peaks, providing information about the spacing between atomic planes. By measuring these angles and intensities, we can determine the crystal’s lattice parameters and atomic arrangement. Bragg’s Law provides the mathematical foundation for X-ray diffraction for crystal structure determination.
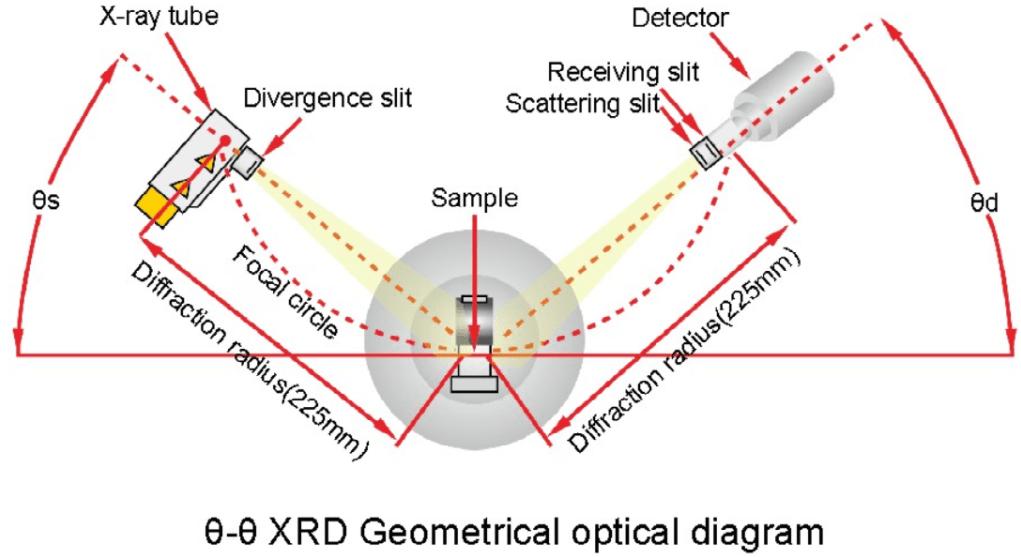
How Does X-ray Diffraction Work for Crystal Analysis?
X-ray diffraction works through a systematic process that transforms X-ray interactions with a crystal into meaningful structural data. Here is a detailed step-by-step explanation:
The sample can be a single crystal or a powder. Powder samples are preferred for routine analysis because they contain many randomly oriented crystallites, ensuring that all possible diffraction angles are sampled.
For powder samples, the material is finely ground to ensure a random distribution of crystal orientations.
The sample is then mounted on a sample holder, often a glass slide or a specialized holder for precise positioning.
- X-ray Generation:
An X-ray tube generates X-rays, typically using a copper or molybdenum target.
The X-rays are collimated (focused) into a narrow beam to ensure a well-defined incident angle.
- Diffraction:
The X-ray beam is directed onto the sample.
The X-rays interact with the atoms in the crystal lattice, causing them to scatter in various directions.
Constructive interference occurs at specific angles, as defined by Bragg’s Law, resulting in intense diffraction peaks.
- Detection:
A detector, such as a scintillation counter or a solid-state detector, measures the intensity of the diffracted X-rays as a function of the diffraction angle (2θ).
The detector moves along an arc, scanning the diffraction angles to capture the entire diffraction pattern.
- Data Analysis:
The collected diffraction data, a plot of intensity versus 2θ, is analyzed using specialized software.
The positions and intensities of the diffraction peaks are used to determine the crystal’s lattice parameters, symmetry, and atomic positions.
For example, the Scherrer equation is utilized to calculate crystallite size from peak broadening.
Databases like the International Centre for Diffraction Data (ICDD) are used to match the observed diffraction pattern with known crystal structures.
Rietveld refinement is a powerful technique to refine the crystal structure model against the entire diffraction pattern, providing accurate atomic positions and structural parameters.
- Structure Determination:
By analyzing the diffraction data, we can determine the unit cell dimensions, the space group (symmetry), and the atomic coordinates within the unit cell.
This information provides a complete three-dimensional picture of the crystal structure.
The step-by-step process of XRD, enhanced by advanced software and databases, allows researchers to obtain detailed crystal structure information.

What Crystals Can XRD Be Used For?
XRD is a versatile tool capable of analyzing a wide range of crystalline materials, including:
- Inorganic Crystals: Minerals, metals, ceramics, and semiconductors are commonly studied to understand their mechanical, electronic, and thermal properties. For example, XRD was crucial in determining the structure of silicon, a cornerstone material in electronics.
- Organic Crystals: XRD helps analyze organic compounds, including pharmaceuticals, pigments, and polymers. The discovery of the double-helix structure of DNA by Watson and Crick in 1953 relied on XRD data.
- Biological Macromolecules: Proteins, enzymes, and other macromolecules can be studied using advanced XRD techniques. For instance, XRD has been instrumental in determining the structures of vital proteins such as hemoglobin and insulin.
- Nanomaterials: XRD is also effective for characterizing nanocrystals and thin films, crucial in nanotechnology and materials science.
By analyzing these diverse crystals, XRD provides insights into material properties and guides innovations in fields ranging from medicine to renewable energy.
X-ray diffraction is an indispensable tool for revealing the hidden crystal structures that underpin the properties of materials. By exploiting the interaction of X-rays with the atomic lattice, XRD provides detailed information about crystal symmetry, lattice parameters, and atomic positions. From mineral identification to pharmaceutical development, XRD plays a crucial role in advancing our understanding of the solid state. As technology advances, XRD techniques continue to evolve, offering even greater precision and efficiency in crystal structure determination. The ability to visualize and understand the atomic arrangement in crystals remains essential for innovation and discovery across numerous scientific and technological fields.



