pH meters are essential instruments used in laboratories, industries, and research facilities for measuring the acidity or alkalinity of a solution. However, like any precision instrument, they can experience errors that affect measurement accuracy. Understanding common pH meter errors and implement effective troubleshooting techniques can help maintain reliable performance and prolong the lifespan of the device.
What are pH Meters
A pH meter is an electronic device used to measure the acidity or alkalinity of a solution, typically expressed as pH, on a scale from 0 to 14. It works by measuring the voltage between a reference electrode and a glass electrode that is sensitive to hydrogen ions in the solution. pH meters are widely used in various fields such as laboratories, industrial processes, agriculture, and water quality testing to ensure proper chemical balance and maintain optimal conditions for different applications.
Common pH Meter Errors and Their Causes
This chart helps identify and understand the common pH meter errors, so they can be addressed effectively.
| Common pH Meter Error | Description | Causes |
| Drifting Readings | The pH meter continuously shifts its reading without any change in the sample. | – Dirty or contaminated electrode – Faulty or improperly calibrated electrode – Electrolyte depletion or contamination |
| Slow Response Time | The pH meter takes longer than usual to stabilize after sample measurement. | – Electrolyte solution dried out – Electrode damage – Low temperature or poor calibration |
| Erratic or Unstable Readings | The readings fluctuate widely or show random values. | – Loose or damaged cable connections – Interference from environmental factors – Faulty electrode |
| Incorrect pH Value | The meter shows pH values that are outside the expected range for the sample. | – Incorrect calibration – Old or expired buffer solutions – Contaminated electrode |
| No Reading | The meter does not display any pH value. | – Damaged electrode – Unstable electrical connections – Low battery in the pH meter |
| High or Low Sensitivity | The meter is too sensitive (overreacts) or not sensitive enough (fails to detect small changes). | – Aged or damaged electrode – Electrolyte depletion – Temperature issues or inadequate calibration |
Effective Troubleshooting Techniques for pH Meter Errors
1. Regular Calibration
Incorrect calibration is one of the primary reasons for pH meter errors. Regular calibration using high-quality buffer solutions is necessary to maintain accuracy. If the readings appear unstable or drift over time, recalibrating with fresh buffers is recommended. Expired or contaminated buffers should be replaced, and the calibration process should be conducted under stable temperature conditions to prevent variations. Using a pH meter with automatic temperature compensation (ATC) further enhances calibration accuracy.
2. Addressing Electrode Contamination
A pH electrode is highly sensitive and can be affected by contamination from previous samples, leading to sluggish or unstable readings. Improper cleaning between measurements can cause buildup on the electrode surface, hindering its ability to respond accurately. Rinsing the electrode thoroughly with distilled water and periodically soaking it in a cleaning solution specifically designed for pH sensors helps remove any deposits. Using tap water for cleaning should be avoided, as it may introduce impurities that can alter readings.
3. Preventing Electrode Drying Out
Proper electrode maintenance is critical for ensuring the longevity and accuracy of a pH meter. If the electrode dries out, it can cause a loss of sensitivity and slow response times. Storing the electrode in a recommended storage solution when not in use helps maintain its hydration and prevents damage. If the electrode has dried out, soaking it in the storage solution for an extended period can help restore its functionality. Using distilled water instead of a storage solution can cause further degradation, reducing the effectiveness of the electrode.
4. Detecting and Resolving Physical Damage
Over time, the electrode may become worn or damaged, leading to erratic readings. Cracks, leaks, or an unresponsive electrode indicate that replacement is necessary. Regular inspection of the electrode for visible signs of wear can prevent unexpected failures during measurement. The reference junction, which facilitates stable readings, can also become clogged, requiring cleaning or replacement if the blockage persists.
5. Minimizing Temperature-Related Errors
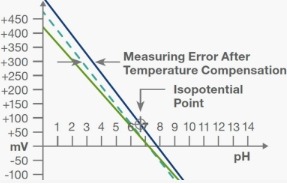
Temperature fluctuations can significantly impact pH measurements, leading to variations in readings. Many pH meters are equipped with automatic temperature compensation (ATC) to correct these variations. If readings appear inconsistent, checking whether the ATC function is active and ensuring that the temperature probe is properly connected can resolve the issue. In cases where manual temperature compensation is required, it is essential to input the correct temperature values to maintain accuracy. Allowing the pH meter to stabilize before taking measurements can further enhance reliability.
6. Reducing Electrical Interference
External electrical noise from nearby equipment can interfere with the pH meter’s readings, causing instability. Instruments such as stirrers, motors, and electronic devices generate electromagnetic interference that affects the pH meter’s signal. Keeping the instrument away from such sources and using shielded cables can reduce the risk of erratic readings. Ensuring proper grounding of the pH meter also helps maintain consistent and accurate measurements.

7. Improving Sample Handling Practices
Errors can also arise from improper sample handling. Ensuring that the sample is well-mixed before measurement prevents inconsistencies caused by varying concentrations within the solution. Rinsing the electrode between different samples minimizes cross-contamination, which can alter readings. It is also essential to avoid air bubbles forming around the sensor, as they can interfere with proper electrode contact and lead to false measurements.

Preventative Maintenance for Preventing pH Meter Errors
- Ensuring Correct Electrode Storage Practices
Storing the electrode correctly is crucial to maintaining its performance. The electrode should never be stored dry, as this can cause dehydration and reduced sensitivity. Instead, it should be kept in a recommended storage solution to prevent drying out. If an electrode has dried out, soaking it in a storage solution before use can help recondition it.
- Inspecting Cables and Connections
Loose or damaged cables can interfere with signal transmission, leading to unstable or fluctuating readings. Regularly checking all connections between the pH meter, electrode, and temperature probe ensures a secure setup. If issues persist, testing with a different electrode or cable may help identify faulty components.
- Battery Maintenance
A low battery can impact the performance of the pH meter, leading to inaccurate measurements or no reading at all. Check and replace batteries as needed, and consider using rechargeable batteries to reduce environmental impact. Always ensure the meter has sufficient power before use.
- Replacing Aging Electrodes
Over time, electrodes degrade and lose sensitivity. Regularly inspect the electrode for wear and tear. If the electrode becomes too slow or unstable, replacing it with a new one is necessary to maintain reliable results. Choose electrodes suitable for the specific applications to improve measurement accuracy.
- Routine Checks and Service
Finally, schedule routine servicing and calibration of the pH meter by professionals to ensure it continues to function properly. This helps identify any potential issues early, preventing costly repairs and ensuring the meter remains accurate over time.
Summary
Troubleshooting pH meter errors involves careful calibration, proper electrode maintenance, and minimizing external interference. Regular inspection and cleaning of the electrode, along with proper storage practices, help maintain the accuracy of pH meters. Through identifying potential error issues and applying appropriate solutions, users can ensure reliable pH measurements, improving efficiency in laboratory and industrial applications.