Fluorescence is the emission of light by a substance after it absorbs light. Fluorescence measurement is a widely used technique in many scientific fields, from biomedical research to environmental monitoring. Its ability to detect and quantify low concentrations of fluorescent molecules makes it highly sensitive and valuable. However, like any analytical technique, fluorescence measurements are susceptible to various errors that can significantly affect the accuracy and reliability of the results. Reducing these errors is essential to ensure precise data, especially in applications where small variations can lead to major differences in conclusions. In this article, we’ll explore the common sources of fluorescence measurement errors and effective strategies to reduce them.

Common Sources of Fluorescence Measurement Errors
Fluorescence measurement errors can arise from a variety of sources, each of which can significantly affect the accuracy and reliability of results. Common sources of errors are:
1. Excitation Source Errors
The excitation source is a critical component of fluorescence measurement systems. It provides the light that excites fluorescent molecules, prompting them to emit light of a different wavelength, which is then measured. Any instability or inconsistency in the excitation source can lead to significant measurement errors.
Key Issues:
Instability of Light Source: Fluctuations in the intensity of the light source can cause variability in the fluorescence signal.
Wavelength Shift: Any slight deviation in the wavelength of the excitation light can affect the efficiency of fluorophore excitation, leading to inconsistent results.
Improper Alignment: If the excitation light is not properly aligned with the sample, it can cause uneven excitation across the sample, skewing the results.
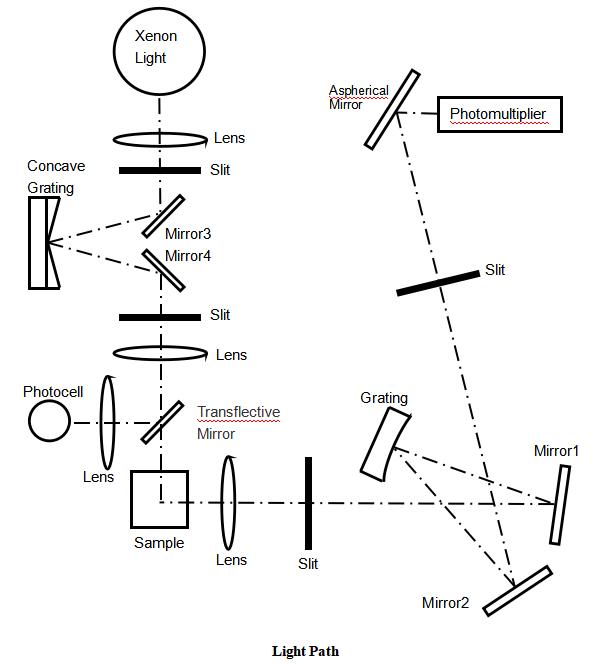
2. Sample Preparation Errors
Proper sample preparation is essential for obtaining reliable fluorescence measurements. Inconsistent or improper preparation can lead to significant errors that affect the reproducibility of the results.
Key Issues:
Concentration Quenching: If the sample concentration is too high, the emitted fluorescence can be quenched, leading to lower-than-expected signals.
Reabsorption of Emitted Light: At higher concentrations, emitted light can be reabsorbed by other fluorophores in the solution, distorting the measurement.
Impurities in the Sample: Contaminants can interfere with the fluorescence signal, either by fluorescing themselves or by absorbing emitted light.
3. Instrumentation Errors
Errors related to the instruments used for fluorescence measurement, such as spectrometers or fluorometers, can have a substantial impact on the accuracy of the results. Instrumental errors often arise from issues like detector sensitivity or calibration problems.
Key Issues:
Detector Sensitivity Variations: Differences in detector response over time or between different detectors can cause inconsistent measurements.
Calibration Drift: Over time, the calibration of fluorescence instruments may drift, leading to systematic errors in the measurements.
Optical Alignment Issues: Misalignment of optical components such as lenses and filters can reduce the efficiency of light detection, leading to weak or skewed signals.
4. Environmental Errors
Environmental factors can also influence fluorescence measurements, introducing variability that may compromise data accuracy. Controlling these factors is vital to reducing errors.
Key Issues:
Temperature Variations: Fluorescence intensity can be affected by temperature changes, as fluorophores may exhibit different behavior at varying temperatures.
Ambient Light Interference: External light sources can interfere with fluorescence detection, leading to background noise or false readings.
pH and Solvent Effects: Changes in the pH or the nature of the solvent can alter the fluorescence properties of the sample, affecting the results.

Strategies to Reduce Fluorescence Measurement Errors
To obtain reliable fluorescence measurements, it is essential to address the potential sources of error through preventive strategies. These strategies involve both technical adjustments and best practices in experimental design.
Reduce Excitation Source Errors
- Use Stable Light Sources: Ensure that the excitation light source, such as a laser or xenon lamp, is stable and reliable. Modern instruments are equipped with more stable light sources, so upgrading to a newer system can help minimize fluctuations.
- Regularly Calibrate the Light Source: Calibrate the excitation source regularly using a known standard to ensure that its intensity and wavelength output remain consistent over time.
- Maintain Proper Optical Alignment: Check and adjust the alignment of the excitation light path to ensure that it is properly directed at the sample. Misalignment can lead to uneven excitation and inconsistent results.
- Monitor Power Output: Use a power meter to monitor the intensity of the excitation light before each measurement to ensure it remains within the desired range.
- Use Optical Filters or Monochromators: Filters or monochromators should be used to precisely select the desired excitation wavelength, avoiding spectral overlap that could reduce the effectiveness of excitation.
Reduce Sample Preparation Errors
- Optimize Concentration: Use the correct fluorophore concentration to avoid concentration quenching or reabsorption effects. Ideally, the concentration should be within the linear range of the fluorometer.
- Minimize Contaminants: Ensure the sample is free from contaminants or impurities that can interfere with the fluorescence signal. This can be done by filtering the sample or using high-purity reagents.
- Prepare Samples Consistently: Follow consistent protocols for sample preparation to ensure that all measurements are comparable. Small variations in sample handling can lead to inconsistent results.
- Choose Appropriate Solvents: Use solvents that do not absorb in the excitation or emission wavelength range, and ensure that solvent fluorescence does not interfere with the sample’s signal.
- Prevent Sample Degradation: Store samples properly and avoid exposure to light, heat, or oxygen, which may degrade the fluorophore. Use freshly prepared samples whenever possible to avoid degradation over time.

Reduce Instrumentation Errors
- Calibrate the Instrument Regularly: Regular calibration of the instrument using a reference standard is crucial to ensure accurate fluorescence measurements. Calibration checks should account for detector sensitivity, wavelength accuracy, and intensity.
- Perform Routine Maintenance: Clean optical components such as cuvettes, lenses, and filters to avoid contamination or damage that can affect the signal. Replace worn-out parts, such as lamps, before their performance deteriorates.
- Check Detector Sensitivity: Verify that the detector’s sensitivity remains consistent by running periodic sensitivity tests. Replace detectors that show signs of aging or instability.
- Use Appropriate Detector Settings: Ensure that the gain and integration time settings on the detector are optimized for the specific fluorophore being measured to avoid saturation or weak signals.
- Eliminate Stray Light and Scattering: Use proper filters and optical setups to eliminate stray light and reduce scattering, which can lead to high background noise in measurements.
Reduce Environmental Errors
- Maintain Stable Temperature: Use temperature-controlled sample holders or incubators to maintain a constant temperature during measurements. Temperature variations can alter fluorescence intensity, especially for temperature-sensitive fluorophores.
- Minimize Ambient Light Interference: Conduct measurements in a dark environment or use a shielded instrument to prevent ambient light from interfering with the fluorescence signal.
- Control Humidity and Air Quality: Avoid dust and particles in the air that can scatter light. Humidity control may also be necessary for sensitive fluorophores or in high-precision measurements.
- Control pH and Solvent Conditions: Ensure that the pH and solvent composition of the sample are carefully controlled and consistent between experiments, as they can influence fluorescence properties.
- Use Proper Cuvettes: Choose the right cuvettes or sample holders that minimize reflections and scattering. For example, quartz cuvettes are often preferred for their transparency and durability in the UV-visible range.
Fluorescence measurement errors can significantly impact the accuracy and reliability of experimental results. Proper calibration, optimized experimental conditions, and routine maintenance are essential strategies to ensure the accuracy and reliability of fluorescence measurements. By understanding the common sources of errors and implementing appropriate strategies, researchers can minimize their impact and obtain more accurate and precise fluorescence data.