Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) is a powerful analytical technique widely used for the detection of trace metals in various samples. Despite its versatility and robustness, achieving low detection limits for trace metal analysis remains a critical focus, particularly in applications where ultra-trace level accuracy is essential. This article explores the key challenges in achieving low detection limits in ICP-OES and strategies to enhance its sensitivity and performance.

What is ICP-OES Spectroscopy
Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) is an analytical technique used to detect and quantify trace elements in a wide variety of samples. It works by introducing a sample into a high-temperature plasma, where the elements are excited to emit light at characteristic wavelengths. The emitted light is then measured by a spectrometer to determine the concentration of each element. Known for its high sensitivity, multi-element capability, and speed, ICP-OES is widely applied in environmental monitoring, material analysis, food safety, and industrial quality control.
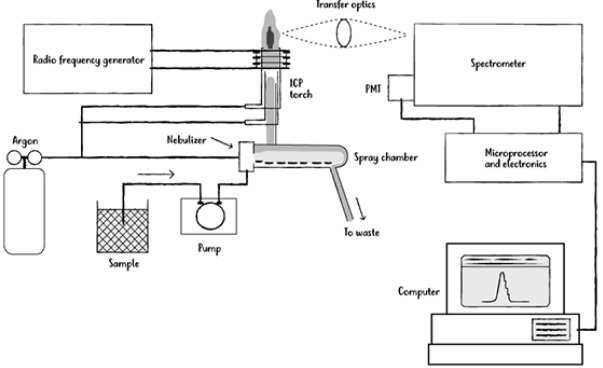
Understanding Detection Limits in ICP-OES
The detection limit is the lowest concentration of an analyte that can be reliably detected above the baseline noise. In ICP-OES, detection limits are influenced by factors such as instrument design, sample preparation, matrix effects, and operating conditions. For trace metal analysis, achieving lower detection limits ensures the accurate quantification of elements present at minute concentrations.

Challenges in Achieving Low Detection Limits in ICP-OES
This structured view provides clarity on the main obstacles faced in achieving low detection limits during ICP-OES analysis.
| Challenge | Impact | |
| Spectral Interferences | Overlapping spectral lines from matrix elements or other analytes obscure target element signals. | Reduces accuracy and sensitivity of measurements. |
| Matrix Effects | Complex matrices or high dissolved solids suppress or enhance emission signals unpredictably. | Compromises reliability and reproducibility. |
| Instrumental Noise | Detector noise or plasma instability lowers the signal-to-noise ratio, obscuring weak signals. | Makes detection of trace levels more difficult. |
| Sample Dilution | Dilution of samples during preparation decreases analyte concentration, complicating detection. | May result in analyte falling below detection limits. |
| Inadequate Nebulization | Inefficient sample aerosol generation leads to weak signals reaching the plasma for excitation. | Lowers signal intensity, affecting sensitivity. |
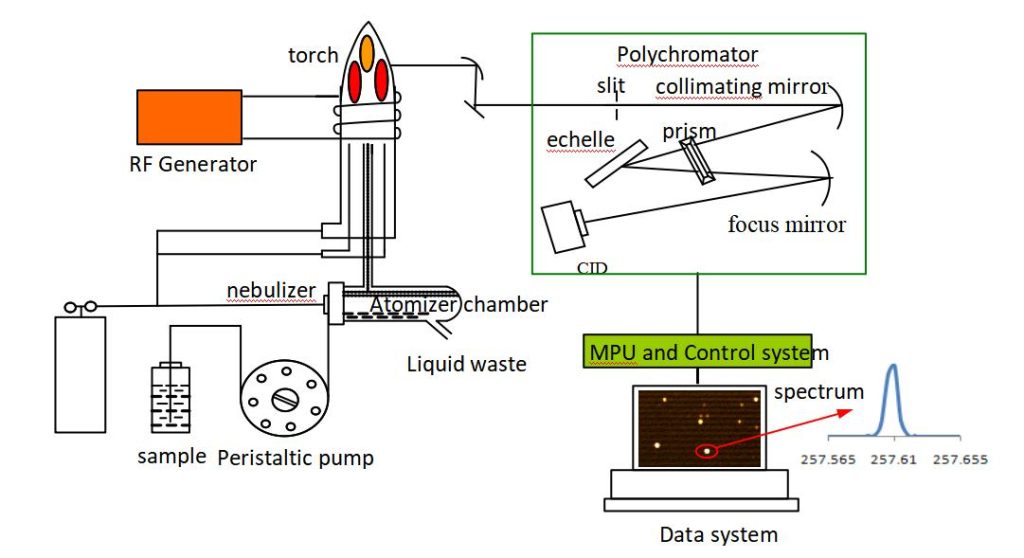
Strategies for Enhancing Detection Limits in ICP-OES for Trace Metal Analysis
1. Optimizing Sample Preparation
- Matrix Matching: Prepare standards and samples with similar matrices to minimize matrix effects.
- Dilution: Dilute samples to reduce interference from high concentrations of other elements.
- Preconcentration Techniques: Use methods like solvent extraction, ion exchange, or co-precipitation to concentrate trace metals before analysis.

2. Improving Plasma Conditions
- Optimizing RF Power: Adjust the radio frequency (RF) power to achieve better excitation and minimize matrix effects.
- Selecting the Appropriate Nebulizer and Spray Chamber: Use high-efficiency nebulizers (e.g., micronebulizers) and spray chambers to improve sample transport and aerosol generation.
- Tuning Argon Flow Rates: Optimize carrier and auxiliary gas flow rates to stabilize the plasma and improve signal intensity.
1. Selecting Suitable Emission Lines
- Choose Lines with Low Background Interference: Select spectral lines with minimal interference from other elements or molecular bands.
- Use Alternative Lines: If interference occurs, use alternative wavelengths for the same element with higher sensitivity.
2. Minimizing Interferences
- Spectral Interferences: Use software tools for spectral deconvolution or use high-resolution ICP-OES systems to distinguish overlapping peaks.
- Matrix Effects: Apply internal standards to correct for signal suppression or enhancement caused by the sample matrix.
5. Reducing Background Noise
- Blank Subtraction: Use high-purity reagents and perform blank corrections to minimize background noise.
- Improve Room Conditions: Maintain a controlled lab environment to minimize fluctuations in temperature and humidity that may affect readings.
6. Regular Instrument Maintenance
- Clean Torches and Nebulizers: Prevent contamination that could increase noise or interfere with signals.
- Monitor Optics and Detectors: Regularly clean and align optical components to maintain instrument performance.
Advancements Driving Enhanced Detection Limits in ICP-OES for Trace Metal Analysis
In recent years, advancements in technology and instrumentation have significantly improved the detection limits of ICP-OES, making it an even more effective tool for trace metal analysis.
- Axial View Plasma Configuration: Axially viewed plasma systems provide a longer optical path, increasing the intensity of emission signals from trace elements. This configuration reduces matrix interferences and improves sensitivity compared to radial view setups.
- High-Resolution Spectrometers: ICP-OES spectrometers combined with advanced detectors like CCD or CID offer higher resolution, allowing for better separation of closely spaced emission lines. This minimizes spectral interferences, a critical factor in achieving low detection limits.
- Improved Sample Introduction Systems: High-efficiency nebulizers (e.g., ultrasonic or concentric nebulizers) and desolvation systems enhance aerosol generation and reduce signal loss. Inert sample introduction systems made from materials like PFA or quartz minimize contamination and enable analysis of reactive trace metals.
- Enhanced Plasma Stability: Developments in plasma generators provide greater stability, reducing baseline noise and enabling detection of ultra-trace concentrations.
- Advanced Software Algorithms: Modern data processing tools incorporate baseline correction, interference modeling, and signal integration techniques, significantly improving detection limits. Automated wavelength selection and interference correction reduce human error and optimize performance.
- Preconcentration Techniques: Coupling ICP-OES with preconcentration methods, such as chelation or ion exchange, increases the concentration of trace elements, enhancing detection capabilities.
- Matrix-Matched Calibration Standards: Improved calibration techniques using matrix-matched standards reduce matrix effects, leading to more reliable trace metal quantification.
- Miniaturized and Portable Systems: Advances in instrument design have led to compact ICP-OES systems capable of delivering comparable sensitivity, making trace metal analysis accessible in field applications.
- Integration with Hybrid Technique: Combining ICP-OES with complementary methods like ICP-MS provides a synergy of sensitivity and specificity, especially for ultra-trace element analysis.
- Environmental and Energy Efficiency: New plasma torches and energy-efficient designs reduce operational costs and improve long-term performance without compromising sensitivity.

To sum up, enhancing detection limits in ICP-OES is critical for trace metal analysis across various industries. Through addressing challenges such as spectral interferences, matrix effects and instrumental limitations, analysts can achieve greater sensitivity and accuracy. With advancements in instrumentation, sample preparation techniques, software tools, hybrid technique, etc, ICP-OES spectroscopy continues to evolve as a reliable method for detecting trace metals at ultra-low concentrations. These strategies not only improve analytical performance but also expand the application scope of ICP-OES in solving complex analytical problems.


2.jpg)