Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is a powerful analytical technique that has become essential in pharmaceutical quality control. Its ability to detect and quantify trace and ultra-trace levels of elements makes it a critical tool for ensuring the safety, efficacy, and compliance of pharmaceutical products with stringent regulatory standards.

Key Applications of ICP-MS in Pharmaceutical Quality Control
1. Elemental Impurity Testing in Active Pharmaceutical Ingredients (APIs)
One of the primary applications of ICP-MS in pharmaceutical quality control is the detection and quantification of elemental impurities in APIs. Regulatory guidelines, such as ICH Q3D, USP <232>, and USP <233>, outline strict limits for elements like arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg) in pharmaceutical ingredients. ICP-MS is highly effective for testing these trace contaminants, as it can detect elements at parts-per-trillion (ppt) levels, ensuring that the concentration of potentially harmful metals remains within safe limits.
2. Excipients Testing
Excipients—inactive substances used in drug formulations—must be free from contamination by elemental impurities. Since excipients are often derived from natural sources or synthesized using various reagents, testing them for elemental content is critical. ICP-MS spectrometer is employed to ensure excipients meet purity standards and do not contain toxic levels of metals that could compromise the safety of the final product.
3. Finished Product Testing
Quality control of the final pharmaceutical product is a vital part of ensuring compliance with regulatory standards. ICP-MS is used to analyze finished products, testing for the presence of metal contaminants from manufacturing processes, equipment, or packaging. It can identify elements that might remain as residual metal catalysts from the synthesis process or those introduced during formulation. By detecting these contaminants, ICP-MS helps ensure that finished products are safe for consumption.
4. Stability Studies and Shelf-Life Testing
During stability studies, ICP-MS is used to track the degradation of products over time. Some pharmaceutical products may degrade into new compounds, potentially including elemental impurities. By using ICP-MS, manufacturers can monitor these changes and assess whether the product remains within acceptable limits for elemental contamination, even during extended storage. Stability studies help determine a product’s shelf life and ensure its safety throughout its use.
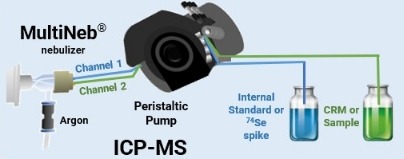
5. Biopharmaceutical Applications
In biopharmaceuticals, such as protein-based therapeutics and vaccines, controlling elemental impurities is even more critical due to the complex nature of biological matrices. ICP-MS is employed to detect trace metals in proteins, antibodies, and vaccines. For instance, it can identify contaminants that could affect the stability or efficacy of biologic products, ensuring they meet both safety and therapeutic standards.
6. Detection of Nutrients and Trace Elements
In some pharmaceutical formulations, especially those designed to provide nutritional supplementation (such as vitamins or minerals), ICP-MS can be used to quantify essential trace elements like zinc, iron, and magnesium. Ensuring the accurate measurement of these elements is vital to maintaining the effectiveness and safety of nutritional supplements or drugs formulated to treat mineral deficiencies.

Benefits of Using ICP-MS in Pharmaceutical Quality Control
- Unmatched Sensitivity: ICP-MS can detect metals at extremely low concentrations, even at the parts-per-trillion (ppt) level, making it ideal for identifying trace impurities in pharmaceutical products that could be harmful if left undetected.
- Simultaneous Multi-Element Detection: One of the key advantages of ICP-MS is its ability to measure multiple elements in a single analysis. This saves time and increases throughput in quality control laboratories, as different elements can be tested together, streamlining the overall process.
- Broad Dynamic Range: ICP-MS has a wide dynamic range, allowing it to measure both trace levels of impurities and higher concentrations of key ingredients or contaminants, all in one test.
- Regulatory Compliance: ICP-MS is recognized by regulatory bodies like the FDA, EMA, and other global authorities as a standard method for elemental impurity testing, ensuring pharmaceutical products comply with the latest safety and quality regulations.
- High Throughput: ICP-MS is capable of delivering rapid results, making it well-suited for high-throughput laboratories that require the analysis of large numbers of samples in a short period.

Challenges and Solutions in ICP-MS Applied in Pharmaceutical Quality Control
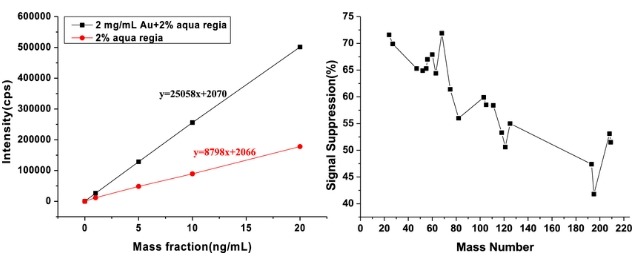
Challenge 1: Matrix Interference
Pharmaceutical samples, especially those containing complex matrices like excipients or biological samples, can produce interferences that affect the accuracy of elemental detection. Matrix effects may lead to signal suppression or enhancement, complicating the analysis of trace elements.
Solution
- Use of Collision/Reaction Cells: Modern ICP-MS instruments are equipped with collision/reaction cells that help reduce matrix interference by removing unwanted ions or interferences from the sample before they reach the detector.
- Standard Addition Method: This technique involves adding known quantities of an element to the sample and comparing the response to the original sample, helping correct for matrix effects.
Challenge 2: Contamination During Sample Preparation
Contamination during the sample preparation stage can lead to inaccurate results. Even trace amounts of contamination from laboratory equipment, reagents, or improper handling can significantly affect the analysis, particularly at the ppt level.
Solution
- Use of Cleanrooms and Dedicated Equipment: To minimize contamination, it is essential to use cleanroom environments and dedicated glassware, tools, and reagents.
- Use of High-Purity Reagents: Ensure that all reagents and solvents used in sample preparation are of high purity and free from metal contamination.
- Blank Samples: Regularly analyze blank samples to detect any potential contamination during the preparation process.
Challenge 3: Sample Preparation Complexity
Pharmaceutical samples, especially those with complex matrices (e.g., tablets, gels, biological samples), require thorough and often complex ICP-MS sample preparation methods, such as acid digestion, to break down the matrix and release analytes for ICP-MS analysis.
Solution
- Automated Sample Preparation: Utilizing automated systems for digestion and extraction can reduce human error and improve reproducibility, efficiency, and throughput.
- Microwave-Assisted Digestion: This technique provides a more efficient and consistent method for digesting samples compared to traditional digestion methods, reducing the chances of incomplete sample preparation.

Challenge 4: Calibration and Sensitivity Issues
Accurate calibration is crucial for reliable ICP-MS analysis. Inconsistent calibration or calibration drift can lead to incorrect measurements, especially when analyzing at low concentrations.
Solution
- Regular Calibration Checks: Conduct regular calibration checks using certified reference materials (CRMs) to ensure the ICP-MS system remains calibrated over time.
- Use of Internal Standards: Internal standards, which are added to samples to compensate for variations in the instrument or sample matrix, can help ensure greater accuracy and precision in the results.
Challenge 5: High Throughput Demands
Pharmaceutical quality control labs often require high-throughput testing of multiple samples. ICP-MS is a highly sensitive technique, but running a large number of samples may be time-consuming and labor-intensive.
Solution
- Automated Systems: Integration of automated sample introduction systems can significantly improve throughput and efficiency by reducing manual sample handling time.
- Optimization of Method Protocols: Streamlining and optimizing ICP-MS methods to handle higher sample volumes while maintaining accuracy can help address throughput challenges.
Challenge 6: Regulatory Compliance and Validation
Pharmaceutical quality control labs must adhere to stringent regulatory requirements set by organizations like the FDA, EMA, and ICH. Ensuring that ICP-MS methods meet these standards and are validated for accuracy, precision, and robustness can be resource-intensive.
Solution
- Method Validation: Ensure that ICP-MS methods are thoroughly validated for parameters such as accuracy, precision, detection limits, and robustness before they are used in routine testing.
- Documentation and Auditing: Maintain thorough records of calibration, method validation, and quality assurance procedures to ensure compliance with regulatory standards and to pass external audits.
Challenge 7: Data Interpretation and Complexity
The complexity of the data generated by ICP-MS, particularly when analyzing multiple elements at trace levels, can make data interpretation challenging. Incorrect data analysis or failure to account for potential interferences can lead to misinterpretation of results.
Solution
- Advanced Data Processing Software: Using specialized software to handle data analysis can simplify the interpretation of ICP-MS results, accounting for interferences and enhancing the accuracy of elemental quantification.
- Regular Training: Ensuring that laboratory personnel are well-trained in both the instrument’s operation and data interpretation helps reduce human error.
Challenge 8: Instrument Maintenance and Calibration Drift
Challenge
ICP-MS instruments are sensitive and require regular maintenance. Instrumental drift, wear and tear, or clogging can affect results if not monitored regularly.
Solution
- Routine Maintenance: Regular preventive maintenance is crucial to keep the ICP-MS in optimal working condition. This includes checking and replacing parts like the torch, cones, and vacuum pumps, as well as cleaning the system to prevent contamination.
- Regular Performance Checks: Periodic checks of instrument performance, including sensitivity, stability, and detection limits, are necessary to ensure reliable results.

Conclusion
ICP-MS provides unmatched sensitivity and precision for detecting elemental impurities in APIs, excipients, finished products, and even biopharmaceuticals in pharmaceutical quality control. Its ability to simultaneously measure multiple elements, coupled with its high throughput, makes it a valuable tool for maintaining product quality and ensuring regulatory compliance.