Gas chromatography (GC) is a powerful analytical technique for separating, identifying, and quantifying the components of complex mixtures. In this article, we will discuss the topic of how does gas chromatography work, exploring its working process and factors affecting separation in gas chromatography.

What is the Working Process of Gas Chromatography?
The principle of selective partitioning between a stationary phase and a mobile phase underpins gas chromatography. Gas chromatography (GC), a highly versatile analytical technique used for the separation and analysis of complex mixtures, is built around the chromatographic process.
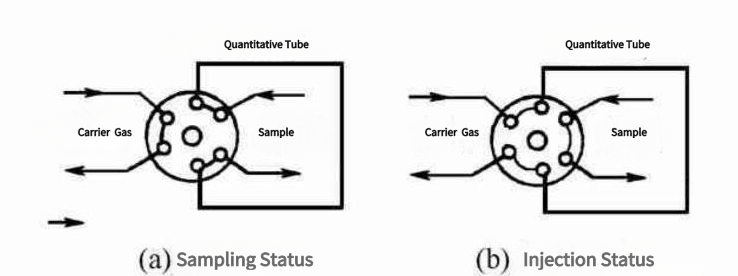
1. Sample Injection
The process begins with the addition of a small amount of sample to the gas chromatograph. Typically, the sample is liquid or gaseous. Solid samples can be vaporized before injection in some cases.
2. Vaporization
The injected sample is vaporized in the GC’s injection port. This is crucial because the column of the GC operates with gases or volatile compounds, and vaporization ensures that the sample can be carried through the column by the mobile phase (the carrier gas).
3. Carrier Gas Flow
The carrier gas used in gas chromatography, which is usually an inert gas such as helium or nitrogen, flows continuously through the system. It functions as the mobile phase, transporting the vaporized sample into the column. The flow rate of the carrier gas is carefully controlled and optimized for the specific analysis.
4. Column Separation
The column is at the heart of the chromatographic process. The column is a long, narrow tube that is typically packed or coated with a stationary phase. The stationary phase is a material that interacts with various compounds differently depending on their chemical properties. The compounds in the sample begin to separate as the vaporized sample is carried into the column by the carrier gas.
Retention Time: Each compound in the sample travels through the column at a different rate. The time it takes for a compound to move from the injection port to the detector is called retention time of gas chromatography. Compounds with different chemical properties will have different retention times due to varying interactions with the stationary phase.
5. Detector Analysis
As the separated compounds exit the column one by one, they enter the detector. The detector is a critical component of the chromatographic process as it identifies and quantifies each compound based on its unique retention time.
Detectors: There are various types of detectors used in gas chromatography, each with its strengths and limitations. Common detectors include:
Flame Ionization Detector (FID): Sensitive to organic compounds and widely used for quantitative analysis.
Mass Spectrometer (MS): Provides both qualitative and quantitative data by measuring the mass-to-charge ratios of ions.
Electron Capture Detector (ECD): Sensitive to compounds containing electronegative elements like halogens and is often used for environmental analysis.
Thermal Conductivity Detector (TCD): Detects changes in thermal conductivity and is useful for detecting compounds that are less volatile.
6. Data Analysis
The detector generates a signal, which is then used to generate a chromatogram. A chromatogram is a graph that depicts the detector’s response over time. Each compound in the sample is represented by a chromatogram peak. The peak’s size corresponds to the concentration of that compound in the sample.
7. Identification and Quantification
Once the chromatogram is generated, the analyst can identify compounds based on retention times, compare them to reference standards, and quantify the amount of each compound in the sample. The area under each chromatogram peak corresponds to the concentration of the corresponding compound.
This chromatographic process is at the heart of gas chromatography’s diverse applications in chemistry, pharmaceuticals, environmental science, forensics, and many other fields.

What are the Factors Affecting Separation in Gas Chromatography?
Compound separation in gas chromatography (GC) is influenced by several key factors that can be controlled and optimized to achieve the desired results. Understanding these variables is essential for obtaining accurate and dependable chromatographic data.
Stationary Phase
The choice of stationary phase is a fundamental factor influencing separation. The stationary phase is a material that lines the interior of the GC column. It can be polar, nonpolar, or specialized for specific applications. The selection of the stationary phase depends on the chemical properties of the compounds in the sample. Polar stationary phases are more effective in separating polar compounds, while nonpolar phases are better for nonpolar compounds.
Column Length
The length of the GC column is an important consideration. Longer columns improve separation capabilities by allowing compounds to spend more time interacting with the stationary phase. Longer columns are better for separating complex mixtures in general, but they can also result in longer analysis times.
Column Diameter
The internal diameter of the column, also known as the column diameter, influences separation efficiency. Narrower columns improve separation efficiency by reducing band broadening, but they may necessitate higher carrier gas flow rates to maintain sample throughput. Wider columns are suitable for analyses requiring higher sample capacity.
Column Temperature
The column temperature plays a critical role in separation. By increasing the temperature, compounds elute (exit the column) more quickly. Conversely, lower temperatures lead to longer retention times. Temperature control is crucial for optimizing separation and achieving desired retention times.

Carrier Gas Flow Rate
The carrier gas flow rate, measured in milliliters per minute (mL/min), influences the speed and efficiency of the separation. Higher flow rates reduce retention times and can decrease the separation resolution, while lower flow rates lead to longer retention times and may improve separation but can also result in longer analysis times.
Sample Injection Technique
Separation can be affected by how the sample is introduced into the GC system. Split injection, splitless injection, and on-column injection are all common injection techniques. The injection method chosen is determined by the sample type, concentration, and desired level of sensitivity.
Column Coating Thickness
The thickness of the stationary phase coating in capillary GC columns influences separation. Thicker coatings interact more with compounds, which can lead to better separation. Thicker coatings, on the other hand, may necessitate higher temperatures and carrier gas flow rates for optimal performance.
Detector Selection
Separation can also be influenced by the type of detector used in gas chromatography. Different detectors respond to different compounds with varying sensitivity and response times. The detector selected should be appropriate for the specific analytical requirements as well as the compounds of interest.
Sample Matrix and Interferences
The sample matrix’s composition can have an effect on separation. Compound co-elution or interference from matrix components can have an impact on chromatographic results. To address these issues, sample preparation techniques such as solid-phase microextraction (SPME) or derivatization may be used.
Column Maintenance
The condition of the GC column is critical. Column contamination or degradation can lead to poor separation. Regular maintenance, including column cleaning and replacement, is essential to ensure consistent and reliable results.

Conclusion
Gas chromatography (GC) can provide valuable insights into the composition of various samples by utilizing the principles of selective partitioning between a stationary phase and a mobile phase, making it an indispensable tool in research, industry, and quality control across diverse fields. Understanding how gas chromatography works is critical for scientists and analysts who want to discover the secrets of molecules in their fields.




