As you know, fluorescence microscopes can be used in different fields. Today, this article will introduce the application of fluorescence microscopes in pathological research.
Components of a Fluorescent Light Microscope
The fluorescent light microscope is made up of the following five parts.
1. Fluorescent dyes: compounds that bind to biomolecules of interest and re-emit light upon excitation (DAPI, Hoechst, Phalloidin).
2. Light source: lasers for complex microscopes. Xenon lamps, mercury lamps, and LEDs with dichroic excitation filters for fluorescence microscopes.
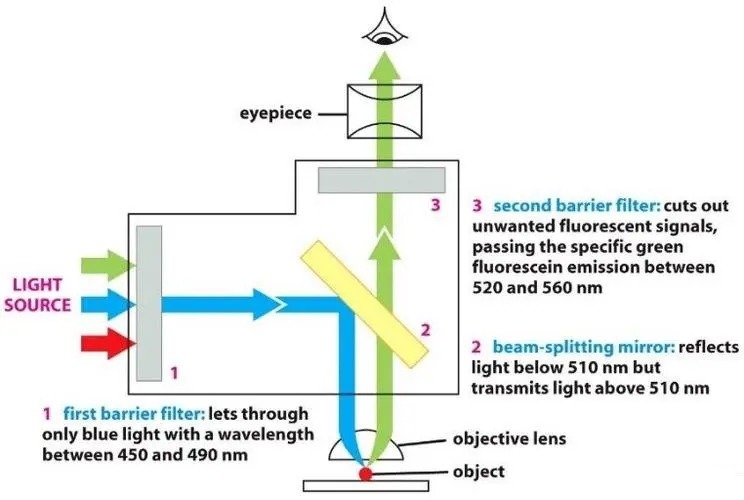
3. Excitation filter: a bandpass filter that only allows the wavelengths absorbed by the fluorophore, minimizing the excitation of other fluorescent sources.
4. Dichroic mirror: a highly specific color filter that only allows a small range of colors and reflects other colors.
5. Emission filter: Bandpass filter only allows the wavelength emitted by the fluorophore and blocks all other unwanted light, especially the excitation light, ensuring the darkest background.
Types of Fluorescence Microscopes
As for the fluorescent light microscope, there are five types.
1. Fluorescence microscope: the transmitted and emitted light passes through the same objective lens.
2. Confocal microscope: combined with optical sectioning, it is specially used for thick 3D samples.
3. Total internal reflection fluorescence (TIRF): Excite a very thin layer of fluorescent molecules under the coverslip.
4. Light sheet microscopy: By using two or more objective lenses to generate a thin layer of excitation light, photobleaching is reduced and 3D imaging is facilitated.
5. Super-resolution microscopy: Imaging at the resolution (diffraction) limit of an optical microscope.
Application of Fluorescence Microscopes in Pathological Research
Fluorescent Microscopes – Applications in Microbiology/Infection Pathology
1. Rapid diagnosis of different fungi, such as yeast, Microsporum, mucormycosis, etc., especially in patients with low immune function.
2. Gram-negative bacteria are more fluorescent than Gram-positive bacteria when a culture fluorescent whitening agent (ANS) is used as an agar medium additive.
Fluorescent Microscopes – Applications in Immunopathological
1. Use fluorescently labeled specific antibodies or antigens to determine the position of antigens or antibodies in tissue sections or smears by fluorescent mode (immunofluorescence).
2. Diagnostic and prognostic tools for autoimmune and vesicular mucocutaneous lesions.
3. Particulate antigens, such as bacteria or protozoa, soluble toxins (staphylococcal enterotoxins), and viruses can be confirmed in tissues or specimens.
4. Identification and titration of host antibodies against infectious agents and autoantibodies against self by indirect fluorescent antibody (immunofluorescence) testing. Unique patterns characteristic of this disease can be seen.
Fluorescent Microscopes – Applications in Cancer Diagnosis
1. Diagnosis of invasive carcinoma manifested as a bright mass with irregular margins consisting of a fluorescent tumor nucleus and surrounding hyperfluorescent fibrous adipose tissue.
2. In high-grade invasive carcinomas, nuclear pleomorphism can be distinguished as irregularly shaped bright spots that are larger than those observed in well-differentiated carcinomas.
3. To determine marginal status in small breast tumors, thyroid lesions (<1 cm), and lymph nodes, where preservation of tissue integrity is important and frozen sectioning is not feasible.
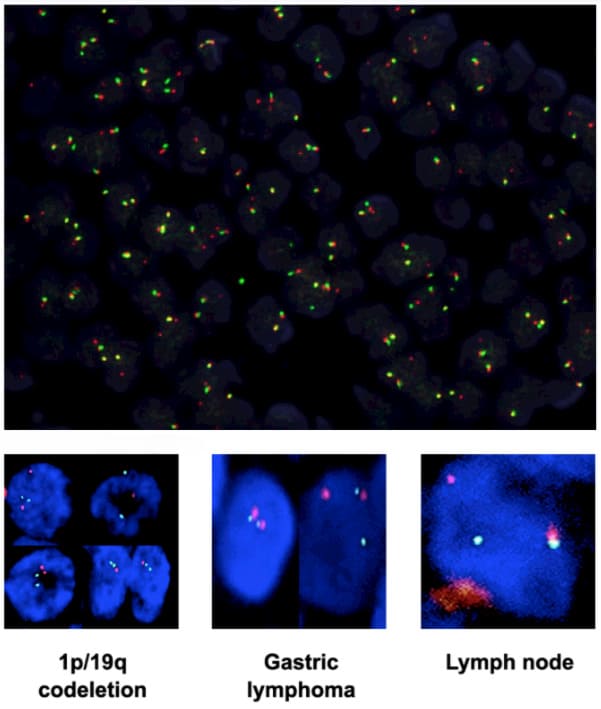
4. Fluorescence in situ hybridization (FISH):
Effectively analyze interphase nuclei, transcription, genes, chromosomal translocations, and small chromosomal abnormalities and assist in the diagnosis of multiple myeloma and leukemia.
An important tool for selecting targeted therapy for leukemia by providing reliable biomarker information.
FISH immunohistochemistry (IHC) to detect protein overexpression and gene amplification (HER2 status in breast cancer).
Fluorescent Microscopes – Application in Research
1. Analyze the intracellular distribution of the nucleus, cell membrane, and various intracellular structures, such as cytoskeleton filaments, mitochondria, and Golgi apparatus.
2. Study living cells and measure the pH value, free calcium, and NAD(P)H concentration in the cytoplasm.
3. Using fluorescent probes to display DNA and RNA sequences in molecular cytogenetics, the fluorescent probes bind to nucleic acid fragments showing a high degree of sequence complementarity.
4. Visualize molecular processes related to bone remodeling in metastatic cancer.
5. Monitoring the virus attachment process and different steps of the virus replication cycle by tryptophan emission spectroscopy analysis.
6. Study the binding affinity of various receptors of viruses.
7. Study the different stages of cell replication, the most common being interphase and metaphase.