Electrophoresis is a chemical process in which charges in a solution flow toward opposite electrodes. In the 1930s, Swedish biophysicist Arne Tisselius developed electrophoresis while studying blood proteins. In 1948, Arne Tisselius was awarded the New Prize in Chemistry for his contribution to the technique of electrophoresis.
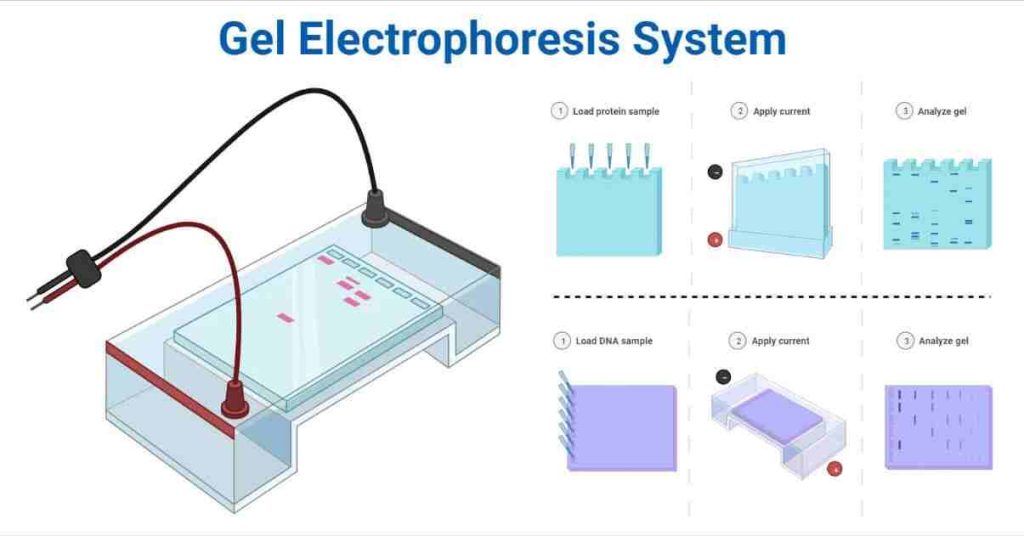
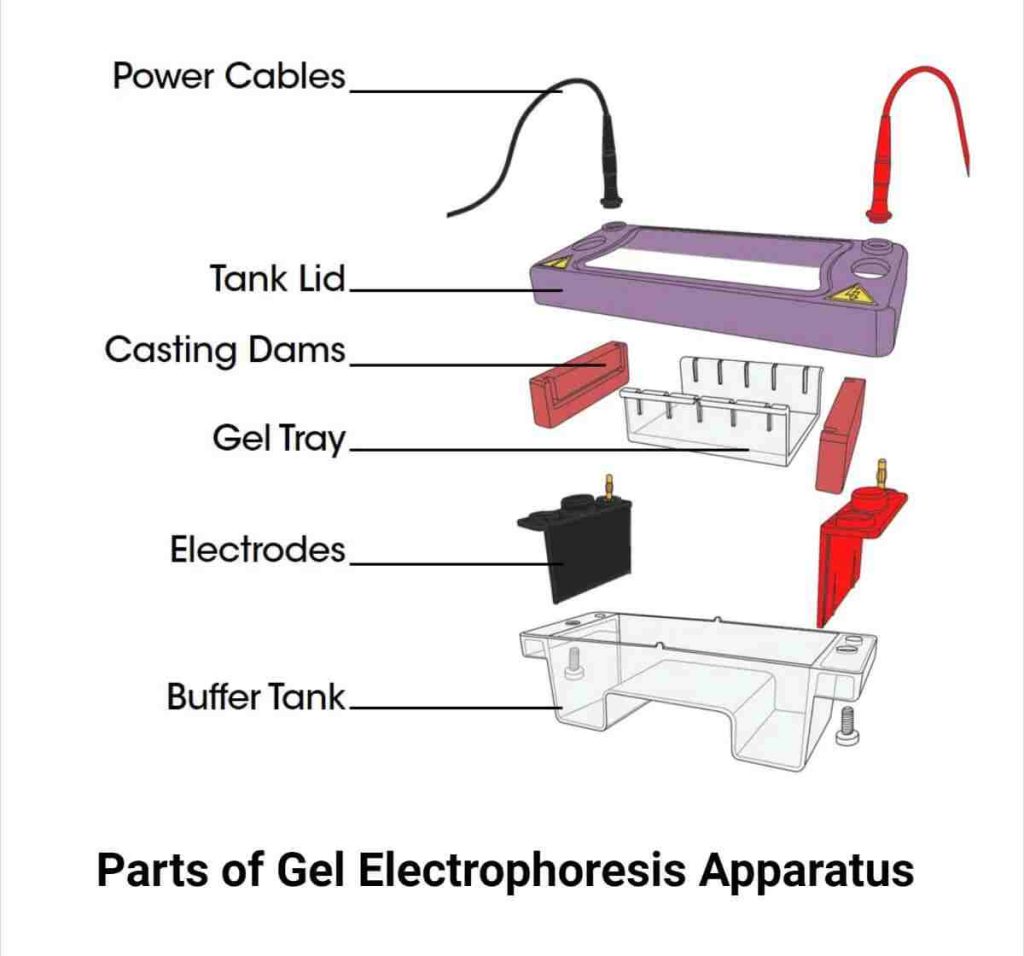
Gel electrophoresis is one of the laboratory methods for separating DNA, RNA, or protein molecules based on their electric charge or size.
How Does Gel Electrophoresis Work?
The principle behind electrophoresis is the observation that most biomolecules exist as charged particles with ionizable functional groups. Depending on the pH, a solution containing biomolecules will have either positively or negatively charged ions.
When charged molecules are placed in an electric field, they travel in the opposite direction to the positive or negative pole. Depending on the mass and net charge of each particle in solution, ionized biomolecules will migrate at different rates when exposed to an electric field.
Negatively charged particles, such as nucleic acids, are drawn toward the anode, while positively charged particles move toward the cathode. Due to changes in speed and direction, each charged particle will migrate in a pattern determined by its specific properties, allowing the separation of biomolecules with similar properties.
Types of Gel Electrophoresis
They are also classified as native and denatured, in which the RNA or protein retains its native structure as it passes through the gel in native gel electrophoresis. In contrast, in denaturing gel electrophoresis, RNA or protein is reduced to a linear structure before or during gel electrophoresis. This reduction is accomplished by adding a reducing agent to the sample, gel, and/or buffer, which cleaves bonds within the RNA or protein molecule and leads to the formation of secondary structures.
paper gel electrophoresis
It is used to study serum and other body fluids in clinical settings.
Opaque and non-toxic
convenient storage
protein adsorption
Poor conductivity
background staining
The OH groups of cellulose attach to proteins and slow down electrophoretic motion, leading to band tailing and poor resolution.
Agarose gel electrophoresis
The concentration of agarose determines the resolution of the electrophoresis.
It is suited for separating DNA fragments ranging from 100 base pairs to 20 kilobase pairs.
Additionally, it applies to the electrophoretic separation of proteins.
When a low concentration of agarose gel is employed, it can be used to separate amphoteric molecules based on their isoelectric point, known as isoelectric focusing.
Polyacrylamide gel electrophoresis (PAGE)
It is used at a concentration of up to 3-30% (pH range: 4-9.0): protein separation requires a higher concentration than DNA separation, and vice-versa.
A greater degree of reliability and accurate porosity.
Its application can be seen in calculating DNA’s molecular weight, DNA sequencing, studying DNA purity, analysis of recombinant DNA molecules and separation of RNA molecules, and measuring the molecular weight of RNA.
Pulsed-field gel electrophoresis (PFGE)
DNA separation in an agarose gel is accomplished by changing the direction and strength of the electrical field between electrodes.
High molecular weight DNA with many megabases or even entire chromosomes are separated using this technique.
PFGE is employed in many fields because it produces precise results that are efficiently reproducible.
It is applied in studies on the molecular biology of pathogens found in food, tracking the genetic stability of organisms employed in the fermentation process, mapping applications such as chromosome rearrangement detection, RFLP, and DNA fingerprinting, and identifying linked strains in the event of hospital outbreaks, etc.
Sodium dodecyl sulfate- Polyacrylamide gel electrophoresis (SDS-PAGE) was Originally called the Laemmli Method after its British inventor U.K. Laemmli.
Upper stacking gel has larger pores with a pH of 6.8, and Lower Separating Gel has smaller pores with a pH of 8.
Proteins are separated based on polypeptide chain length in SDS-PAGE, which largely eliminates the influence of the structure and charge thanks to the use of sodium dodecyl sulfate (SDS, also known as sodium lauryl sulfate) and polyacrylamide gel.
SDS, a detergent in the sample buffer, and some reducing chemicals work together to damage the tertiary structure of proteins by rupturing their disulfide links.
It is used to calculate the protein’s molecular weight and determine whether protein samples are pure or not.
Isoelectric point and Isoelectric focusing (IEF)
The pH level known as the isoelectric point is the one where proteins have no net charge (pI). Proteins are separated by their isoelectric points within a continuous pH gradient using the high-resolution approach known as isoelectric focusing (IEF). Compounds that differ in pI by only 0.01 pH units can be separated thanks to the excellent resolving power.
It is used to distinguish isoenzymes, fractionate proteins, and separate all amphoteric substances.
2D gel electrophoresis
It is used to analyze complicated protein mixtures and was created as a hybrid of the 2DGel, IEF, and SDS-PAGE procedures.
IEF separates the protein into its charges in the first step and later according to its mass in the second step.
SDS treatment makes the separated protein on the IEF gel negatively charged, and the electrophoresis is carried out by placing the gel horizontally inside the SDS-PAGE gel.
As a result, the proteins that are concentrated on the pI are divided based on their molecular weights.
Immuno-electrophoresis (Rocket Electrophoresis)
In the process of immune-electrophoresis (IEP), firstly, electrophoresis is used to separate the protein antigen in semi-solid media, and then an immunodiffusion against the antiserum results in the creation of precipitin.
Suitable antibodies complementary to the test antigen to be measured are dissolved in a molten agar solution and placed on a horizontal plate. Antigens are injected into wells drilled in the gel. Ag picks up a negative charge at the alkaline pH, moves in the anode direction, interacts with Ab to form the Ag-Ab complex, ad precipitates. The immuno-precipitates will then appear as arcs like rockets once the gel has been stained with a suitable dye like CBB.
Application of Gel Electrophoresis
DNA fingerprinting isolates DNA fragments for crime scene investigation and paternity testing.
Detect genetic variants and proteins associated with health and disease.
It is used to detect and purify nucleic acids and proteins for scientific purposes.
It helps find pathogens in sources such as blood, other tissues, or food.
It helps identify and purify proteins or nucleic acids that are often examined in more detail using mass spectrometry or DNA sequencing.
It is used in blotting methods for the analysis of macromolecules and for evolution studies.
It helps to evaluate the results of polymerase chain reaction (PCR).
Both vaccine development and manufacturing benefit from electrophoresis.
To differentiate species and evolutionary relationships, taxonomic-DNA analysis was performed.
Advantages of Gel Electrophoresis
- Affordable.
- Make direct connections between similar outcomes
- easy to execute
- DNA can be tested from any type of evidence.
- excellent resolution
- Available in various pore sizes.
- Stable over a wide range of pH, temperature, and ionic strength
- Translucent
- chemically inert
- Electroneutrality and hydrophilicity




